Translate this page into:
Plasma Cell Infiltration of the Kidney as a Manifestation of Myeloma: A Report of Three Cases
Address for correspondence: Dr. M. D. Padua, Department of Histopathology, Apollo Hospitals, Jubilee Hills, Hyderabad, Telangana, India. E-mail: michelledepadua@hotmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Infiltration of renal parenchyma by neoplastic plasma cells in myeloma patients is an unusual finding. We report 3 cases of myeloma, with renal biopsy being the first clue to the diagnosis in one. The plasma cell infiltrate in other two cases was not so evident but immunofluorescence (IF) and immunohistochemical (IHC) stains for light chains helped establish the monoclonal nature of the infiltrate. We surmise that plasma cell infiltration in the kidney can be an important clue to the diagnosis of an underlying myeloma and could in future be regarded as a myeloma-defining event (MDE) if monoclonality is confirmed. This finding could directly affect the prognosis and be a direct indicator of the tumor burden. Further studies are however required to determine the exact prognostic value and precise relationship of such a finding with deranged renal functions in myeloma.
Keywords
Myeloma
plasma cells
renal infiltration
Introduction
Multiple myeloma (MM) has heterogeneous morphological manifestations, in the context of renal involvement. Infiltration of renal parenchyma by neoplastic plasma cells is rare and usually, occurs in terminal patients with myeloma.[1] Renal insufficiency is a frequent complication of myeloma,[2] and cast nephropathy is the most common cause of acute kidney injury (AKI) in these patients. AKI due to infiltration by neoplastic plasma cells in MM is very rare.[3] Only few cases have been reported in literature.[4567] Previously, it has been reported in various autopsy series of patients with MM.[8910] We report three cases, with a common finding of neoplastic plasma cells infiltration into the renal parenchyma. AKI due to neoplastic plasma cell infiltration per se was seen in one case, while in the other two cases, this was an incidental finding, with cast nephropathy being the most probable cause of AKI. Heterogeneity in all these cases represents the various combinations of different patterns of renal injury we can encounter in MM. At our institution, the updated guidelines of the International Myeloma Working Group are followed to diagnose MM. Details of the laboratories of all the three cases discussed below are summarized in Table 1.

Case Reports
Case report 1
A 72-year-old male, known diabetic and hypertensive since 15 years, presented with generalized weakness for 2 months. Laboratory investigations of the patient are summarized in Table 1.
The single core of renal biopsy showed 11 glomeruli, 2 of which were sclerosed. The viable glomeruli appeared normal except for a mild periodic acid-Schiff (PAS) positive mesangial widening. The striking finding was the dense infiltrate of plasma cells in the interstitium, including mature and immature ones, few with nuclear atypia, involving more than 80% of the cortex sampled, compressing the microvasculature [Figure 1a and b]. There was associated tubular injury, with a flattened lining epithelium. Features of cast nephropathy were not seen.
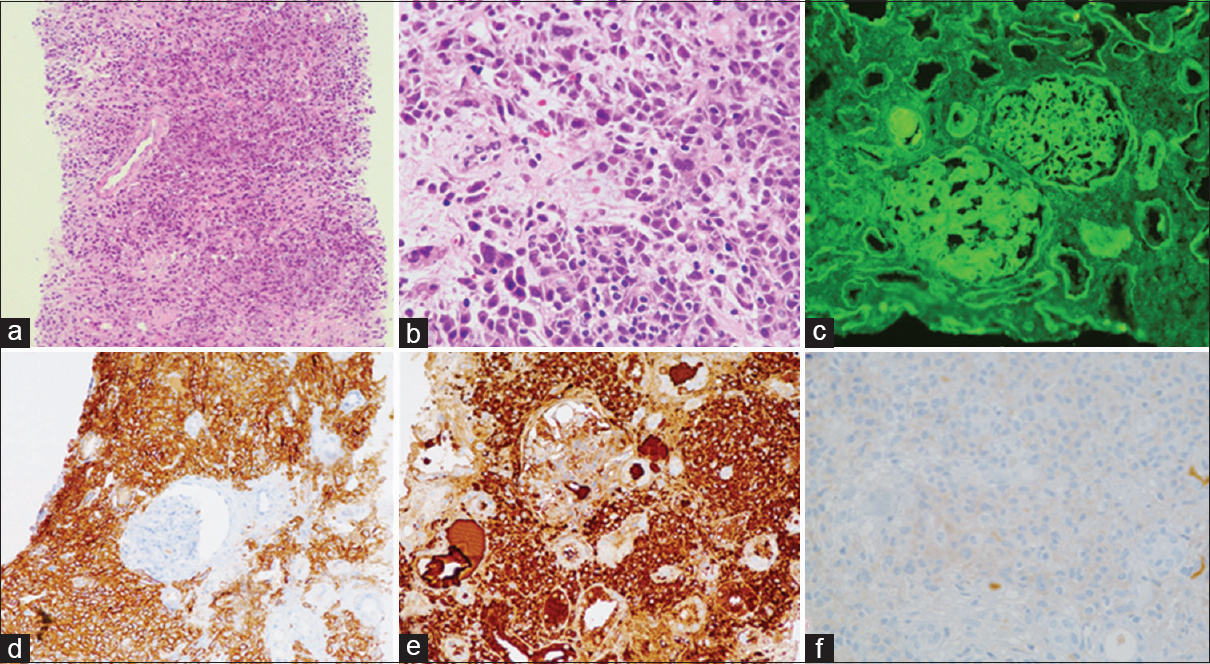
- Case 1: (a) Dense infiltrate of plasma cells in the interstitium, compressing the tubules and microvasculature (×4, H and E). (b) Atypical plasma cells can be appreciated with atypical hyperchromatic nuclei and many binucleated forms (×4, H and E). (c) Immunofluorescence study showing linear deposits of kappa along the glomerular basement membrane, Bowman's capsule and tubular basement membrane. (d) Immunostain for CD138 highlighting the plasma cells. (e) Immunostain for kappa is positive in the neoplastic plasma cells while that for lambda is negative (f)
IF studies showed linear deposits of kappa light chain along the glomerular basement membrane (GBM), Bowman's capsule and tubular basement membrane (TBM) [Figure 1c]. IHC for CD138, kappa, and lambda light chains was done. The plasma cells were positive for CD138 and showed restriction for kappa light chains [Figure 1d–f]. IHC also showed focal granular and linear deposits of kappa light chain along the GBM and TBM. Tubular casts did not show any light chain restriction. On the basis of above histology, the diagnosis of monoclonal plasma cell myeloma infiltrate, with kappa chain restriction was given, with associated light chain deposition disease (LCDD).
Subsequent bone marrow biopsy revealed 90% plasma cells. Lytic lesions were not seen. Serum protein electrophoresis (SPEP) showed a prominent M-band. The patient was referred to a hemato-oncologist for treatment; however, he succumbed to his illness after 15 days due to renal failure.
Case report 2
A 63-year-old male presented with AKI. Semiquantitative analysis by dipstick showed proteinuria of 2+. In view of the M-band on SPEP with renal dysfunction [Table 1], a cast nephropathy was suspected. A renal biopsy was performed to confirm the same.
The biopsy showed a single core of renal cortex with up to 6 glomeruli, one of which was obsolescent. The glomeruli showed a mild basement membrane thickening. Scattered tubules had PAS negative, focally fractured casts, with a histiocytic reaction around them with associated tubular injury [Figure 2a]. Marked interstitial fibrosis with tubular atrophy was noted in more than 50% of the cortex studied. Atrophic tubules and few nonatrophic tubules showed a markedly thickened TBM. Interstitium was widened with a moderately dense infiltrate of plasma cells with admixed lymphocytes. Few of the plasma cells were seen in aggregates and showed prominent nucleoli [Figure 2b]. Arteries showed medial hyperplasia.
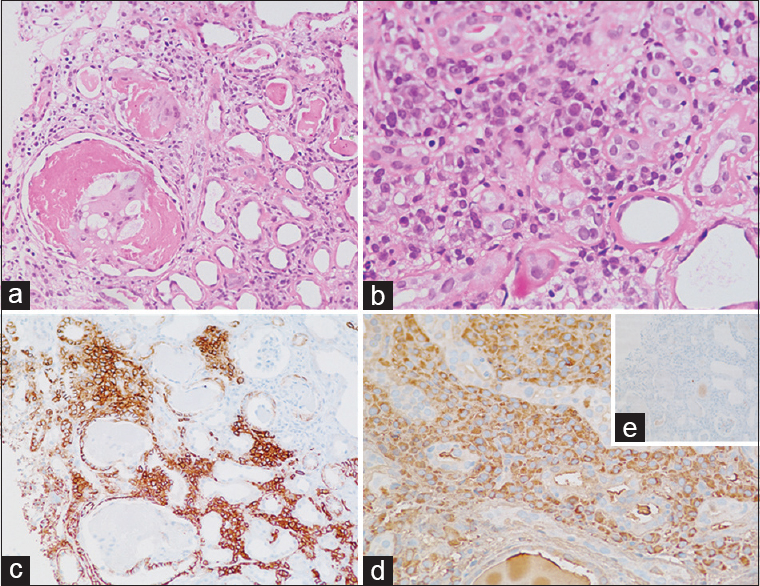
- Case 2: (a) Bright eosinophilic casts in the dilated lumen of tubules inciting histiocytic reaction, and tubules showing features of tubular injury (×20, H and E). (b) Aggregates of atypical plasma cells and tubules showing marked thickening of the tubular basement membrane (×40, H and E). (c) CD138 immunostain highlighting the interstitial plasma cells. (d) Immunostain for lambda revealing lambda restricted plasma cells, and negative kappa (e, inset)
IF showed lambda light chain restriction in the casts along with deposits of the same light chain along the GBM, TBM, and Bowman's capsule. IHC for CD138, kappa, and lambda light chains was done, which showed CD138 positive plasma cells to be lambda light chains restricted [Figure 2c and d].
Based on the above findings, a diagnosis of cast nephropathy was given, with associated LCDD and direct infiltration of neoplastic plasma cells into the kidney.
Subsequently, bone marrow biopsy was done which showed 60% plasma cells. The patient was on hemodialysis for 2 months. Chemotherapy for MM was initialized. His serum creatinine levels came down to 4.6 mg/dl, however, never touched the baseline again. However, he passed away 6 months after starting treatment due to multiple organ failure secondary to sepsis.
Case report 3
A 73-year-old female presented with back pain, pedal edema, and raised serum creatinine [Table 1]. Urine albumin, on dipstick was 2+, with no pus cells or plasma cells on routine microscopy. Renal biopsy showed a core of renal cortex with 3 glomeruli, 2 of which were completely sclerosed. The viable glomerulus showed a mild PAS positive mesangial widening. Few tubules contained PAS negative rigid casts, focally inciting histiocytic giant cell reaction around it. Mild interstitial fibrosis and tubular atrophy was seen in 15%–20% of the cortex studied. Interstitium showed a moderate infiltrate of lymphocytes, few plasma cells, and few neutrophils. Blood vessels were unremarkable.
IF study showed casts with lambda light chain restriction. It also showed focal aggregates of plasma cells in the medullary part of the interstitium, with lambda light chain restriction [Figure 3a]. IHC was done on IF reprocessed tissue. The plasma cell aggregates were positive for CD138 and lambda light chains [Figure 3b]. Interestingly, these plasma cells were evident only on IF tissue. Tissue for light microscopy was also stained for CD138, kappa, and lambda, which showed two small aggregates of plasma cells, which were missed during reporting.

- Case 3: (a) Immunofluorescence for lambda light chains showing clusters of plasma cells picking up fluorescence and an occasional cast with lambda light chain restriction. (b) Lambda chain immunostain done on reprocessed tissue which was submitted for immunofluorescence, showing clusters of plasma cells with lambda chain restriction. Kappa light chains were negative
The serum creatinine came down from 8.4 to 2.2 mg/dl after starting treatment for myeloma cast nephropathy. However, she expired 3 months after the diagnosis due to pneumonia.
Discussion
AKI is a frequent complication of myeloma found in more than 50% cases at initial examination, which was also the scenario in the above three cases. Renal involvement in myeloma is multifaceted. In some instances, more than one renal compartment is affected, with different patterns of renal injury occurring in the same patient.[3] The same was seen in all our cases described above. Apart from the common finding of direct renal infiltration of neoplastic plasma cells, LCDD was seen in case 1 and 2 and cast nephropathy in case 2 and 3.
Direct infiltration of plasma cells into the renal parenchyma is an extremely rare finding and generally occurs in patients with advanced disease. The incidence of plasma cell infiltration in the kidney in myeloma patients was just 3.8% according to one study[10] and 3.6% according to another study,[11] both of which were done on autopsy kidneys of patients suffering from myeloma. The incidence in the patients having myeloma, presenting with renal insufficiency still needs to be ascertained. Direct infiltration of neoplastic plasma cells into the renal parenchyma could directly result in AKI due to the combination of compression of tubules and the microvasculature by the infiltrative process, and local light chain and cytokine-mediated tubular epithelial injury, which was evident in case 1, where the interstitium was packed with plasma cell infiltrate. This pathogenesis is consistent with a similar explanation for renal failure given for other cases with hematolymphoid malignancies.[612] The dense infiltrate of neoplastic plasma cells, as in case 1, and small aggregates of neoplastic plasma cells in case 2 and 3, is likely to be an independent poor prognostic factor, possibly homing the kidney because of favorable microenvironment and increased tumor load in the hematolymphoid organs. It is possible that such a manifestation be regarded as a myeloma-defining event (MDE) in future since monoclonality can be confirmed on the renal biopsy itself. This would be helpful in cases where bone marrow plasma cells are inadequate according to the guidelines, as was in case 3, and hence help in timely initiation of therapy.
IHC and IF uncovered the neoplastic plasma cells in case 2 and case 3, respectively. Both these cases had a mixed inflammatory infiltrate and the neoplastic nature of infiltrating plasma cells was not so apparent. Hence, we suggest that renal biopsy of patients with myeloma, cast nephropathy, LCDD, or any evidence of monoclonal gammopathy should be viewed and analyzed critically on light microscopy for any atypical plasma cells or their aggregates.
Since infiltrating plasma cells may appear benign, IHC for CD138, kappa, and lambda are necessary to ascertain the neoplastic nature of these plasma cells. Plasma cells may not be distributed homogeneously throughout the renal cortex and medulla. They may be present focally in clusters. Hence, it is important to view the entire tissue submitted, including tissue sent for IF, to avoid missing such a rare finding.
To conclude, renal failure in patients with myeloma is multifactorial. There have been many studies on the pathogenesis of various other, more common, renal manifestations of plasma cell dyscrasias. However, the impact of direct kidney infiltration by neoplastic plasma cells still needs to be delineated, since there is paucity of data in literature on this particular manifestation. IF and IHC play an important role to help catch these reactive looking neoplastic plasma cells with light chain restriction, minimizing the chances of missing such a finding, which directly could be an indicator of tumor burden. Displaying monoclonality of plasma cells, be it by IF or IHC, could in future be included in the list of MDE's, and reduce the cost and pain of other interventions.
The study is limited by lack of correlation of plasma cell infiltration in kidney with the tumor burden. Further radiological and molecular studies for estimation of tumor burden and its correlation with the plasma cell infiltration will probably give us a better insight into this subject.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Monoclonal immunoglobulin deposition disease: Light chain and light and heavy chain deposition diseases and their relation to light chain amyloidosis. Clinical features, immunopathology, and molecular analysis. Ann Intern Med. 1990;112:455-64.
- [Google Scholar]
- Renal function in newly diagnosed multiple myeloma – A demographic study of 1353 patients. The Nordic Myeloma Study Group. Eur J Haematol. 1994;53:207-12.
- [Google Scholar]
- Renal diseases associated with plasma cell dyscrasias, amyloidosis, and waldenstrom macroglobulinemia. In: Jennette JC, Olson JL, Silva FG, D'Agati VD, eds. Heptinstall's Pathology of the Kidney (7th ed). Philadelphia: Lippincott Williams & Wilkins; 2015. p. :951-1014.
- [Google Scholar]
- The kidney in multiple myeloma – Medical Staff Conference, University of California, San Francisco. West J Med. 1978;129:41-8.
- [Google Scholar]
- Renal histological lesions and clinical syndromes in multiple myeloma. Renal Immunopathology Group. Clin Nephrol. 1987;27:222-8.
- [Google Scholar]
- Acute renal failure caused by renal infiltration by hematolymphoid malignancy. Ann Diagn Pathol. 2006;10:230-4.
- [Google Scholar]
- Renal failure caused by plasma cell infiltration in multiple myeloma. Clin Exp Nephrol. 2011;15:586-90.
- [Google Scholar]
- Multiple myeloma: A clinicopathologic study of 62 consecutively autopsied cases. Medicine (Baltimore). 1980;59:380-92.
- [Google Scholar]
- Clinical and pathologic findings in 52 consecutively autopsied cases with multiple myeloma. Am J Hematol. 2001;67:1-5.
- [Google Scholar]
- Renal pathologic spectrum in an autopsy series of patients with plasma cell dyscrasia. Arch Pathol Lab Med. 2004;128:875-9.
- [Google Scholar]
- Acute renal failure due to a malignant lymphoma infiltration uncovered by renal biopsy. Nephrol Dial Transplant. 2004;19:2657-60.
- [Google Scholar]







