Translate this page into:
Plasma von Willebrand Factor and a Disintegrin and Metalloproteinase with Eight Thrombospondin-type 1 Motif Levels in Hemodialysis Patients: Relation to Vascular Access Thrombosis
Address for correspondence: Dr. Khaled M. A. Elzorkany, Department of Internal Medicine, Nephrology Unit, Faculty of Medicine, Menoufia University, Menoufia Governorate, Egypt. E-mail: khaledelzorkany1979@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Vascular access complications are major issues in hemodialysis (HD) patients, which increase their morbidity and lessen HD efficiency. The aim of this study was to assess von Willebrand factor (VWF), and a disintegrin and metalloproteinase with eight thrombospondin-type 1 motif (ADAMTS13) levels in HD patients and their association with vascular access thrombosis (VAT). This study included a total of 158 individuals including 128 patients undergoing HD for more than 6 months, subdivided into two groups according to the occurrence of the previous episode of VAT; 60 HD patients with VAT and 68 HD patients without VAT and 30 healthy controls. Plasma ADAMTS13 and VWF levels were assessed by enzyme-linked immunosorbent assay technique. There were higher VWF levels and lower ADAMTS13 in HD patients, compared to healthy controls. Furthermore, VWF levels were significantly higher and ADAMTS13 levels were significantly lower in HD patients with VAT than those without VAT. Further prospective studies with large number of patients are thus needed to show if there is causal relationship between higher VWF levels, lower ADAMTS13, and VAT.
Keywords
A disintegrin and metalloproteinase with eight thrombospondin-type 1 motif
hemodialysis
vascular access thrombosis
von Willebrand factor
Introduction
Patients on hemodialysis (HD) have a risk of vascular access thrombosis (VAT) which increases morbidity.[1] VAT in HD patients is mainly predisposed by vascular narrowing due to fibromuscular and intimal hyperplasia, which result in drop of blood flow through vascular access. This drop of blood flow results in blood stasis, which leads to low blood pressure, blood volume, and hypercoagulable state within fistula. Decreased blood flow and stasis secondary to intimal hyperplasia predispose to thrombosis.[2]
Von Willebrand factor (VWF) is a critical part of the coagulation cascade and can be used as biomarker of hypercoagulation.[3] The VWF is a multifunctional acute-phase glycoprotein with a crucial role in the formation of platelet thrombi. It consists of similar subunits of 270 kDa each composed of 2050 amino acids produced by endothelial cells and megakaryocytes into subendothelium and plasma, respectively, whereas very large VWF is stored in endothelial cells and on injury to endothelium, is released at the site of endothelial injury.[4]
VWF contributes to thrombus formation by two mechanisms, directly by mediating platelet adhesion to subendothelial collagen and indirectly by preventing clearance of Factor VIII (FVIII) from plasma. Hence, elevated levels of FVIII and VWF are strongly linked to atherosclerosis and thromboembolism.[5] Newly secreted ultra large multimeric VWF (UL-VWF) have a high spontaneous platelet binding potential and consequently must be enzymatically degraded into smaller, less thrombogenic units before entering the circulation.[6]
A disintegrin and metalloproteinase with eight thrombospondin-type 1 motif (ADAMTS) family of secreted multidomain zinc metalloproteases cleave the peptide bond between tyrosine at position 1605 and methionine at position 1606, in the central A2 domain of VWF.[7] A member of the ADAMTS family with mass of approximately 150 kDa, named ADAMTS13 (a disintegrin and metalloproteinase with eight thrombospondin type 1 motif, 13), also known as VWF-cleaving protease is a proteolytic enzyme cleaves and removes UL-VWF from circulation, prevents elongation and accumulation of hyper-reactive UL-VWF strands on activated endothelial surface.[8] ADAMTS13 is mainly produced by the liver and circulates in the blood.[9] It has large molecular weight, so it is not affected by the type of dialysis and dialyzer membranes. VWF is the only known substrate for ADAMTS13, so deficiency and/or the presence of antibodies against ADAMTS13 may elevate UL-VWF plasma levels, thus leading to thrombosis in small vessels.[10]
The aim of this study was to assess plasma level of VWF and ADAMTS13 in HD patients and their association with VAT.
Patients and Methods
This cross-sectional study included a total of 158 individuals including 128 HD patients and 30 unrelated healthy, age- and sex-matched individuals as control group. Patients were selected from four dialysis centers in Menoufia governorate, Egypt. The HD patients were subdivided into two groups, according to the occurrence of a previous episode of VAT. The first group included HD patients with VAT (60 patients) who experienced, at least, one previous episode of thrombotic occlusion, which was identified by failure to feel thrill in access and inability to use the access for dialysis. The second group included HD patients without VAT (68 patients) with no history of previous thrombotic occlusion.
All patients had regular HD sessions 3 times per week, for 3–4 h. The dialysate flow rate set at a 400–500 mL/min and blood flow rate set at 300–450 mL/min. They were dialyzed with low-flux polysulfone dialyzers with bicarbonate-based solutions. These patients were heparinized (by unfractionated or low molecular weight heparins), before and during HD session. The following data were obtained from patients; age, gender, etiology of end-stage renal disease (ESRD), body weight, predialysis blood pressure levels, type of vascular access, duration of HD, interdialytic weight gain, and urea reduction ratio (URR). Written consent (approved by the Committee of Human Rights in Research at Menoufia University) was obtained from all studied subjects before blood sample collection for laboratory investigations, which included lipid profile (serum total cholesterol [TC], triglycerides [TG], high-density lipoprotein cholesterol [HDLc], and low-density lipoprotein cholesterol [LDLc]), serum albumin, serum urea, total hemoglobin and plasma VWF and ADAMTS13 levels using standard laboratory techniques.
The URR was calculated from pre- and postdialysis blood urea levels.[11] Plasma VWF levels were measured by enzyme linked immunosorbent assay (ELISA) method, using Thermo-scientific™ human VWF ELISA kit from the USA.[12] Plasma ADAMTS13 levels were determined by ELISA method, using Quantikine® Human ADAMTS13 Immunoassay kit from the USA.[13]
Statistical analysis
Data were collected, tabulated, statistically analyzed by computer using SPSS version 16 (SPSS Inc., Chicago, IL, USA). Data were statistically described as mean ± standard deviation (X ± SD) for quantitative variables and analyzed by applying Student's t-test for comparing two groups of normally distributed variables and as frequency and percentage for qualitative variables. Chi-square was used to compare between two groups of categorical variables. P ≤ 0.05 was considered statistically significant.
Results
This cross-sectional study showed that age and gender were matched between studied groups (P > 0.05) [Table 1]. Clinical data and HD parameters did not differ statistically between HD patients with and without VAT [Table 1]. Body weight, duration of dialysis (years), interdialytic weight gain, predialysis systolic blood pressure, predialysis diastolic blood pressure, URR, hemoglobin, TC, LDLc, HDLc, TG, and serum albumin were not significantly different between both groups (P > 0.05) [Table 1]. There were no statistical differences between both patient groups according to cause of ESRD; 19.3% diabetes mellitus (DM), 14.8% glomerulonephritis (GN), 33.5% hypertension, 2% lupus nephritis, 2.1% polycystic kidney disease (PKD), 29.3% other or unknown causes in HD patients without VAT while in HD patients with VAT; 20% DM, 17.5% GN, 34% hypertension, 1.5% lupus nephritis, 2% PKD, and 25% other or unknown causes.

HD patients had significantly higher plasma VWF levels than healthy controls (P < 0.05) while a significant decrease in plasma ADAMTS13 levels was observed in HD patients, as compared to the control group (P < 0.05) [Table 2 and Figures 1, 2]. In addition, HD patients with VAT showed significantly higher plasma VWF levels and lower ADAMTS13 levels (P < 0.001) than others without VAT [Table 2 and Figures 1, 2].

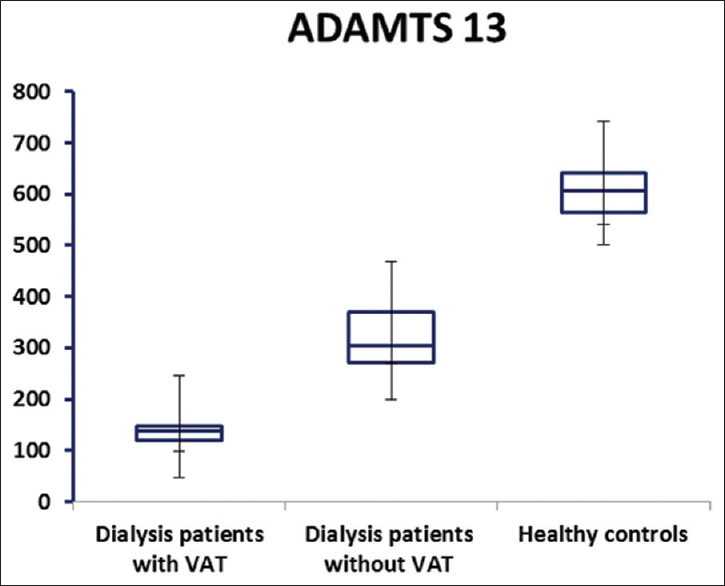
- A disintegrin and metalloproteinase with eight thrombospondin-type 1 motif (ng/ml) plasma levels in dialysis patients with, without vascular access thrombosis and healthy control group
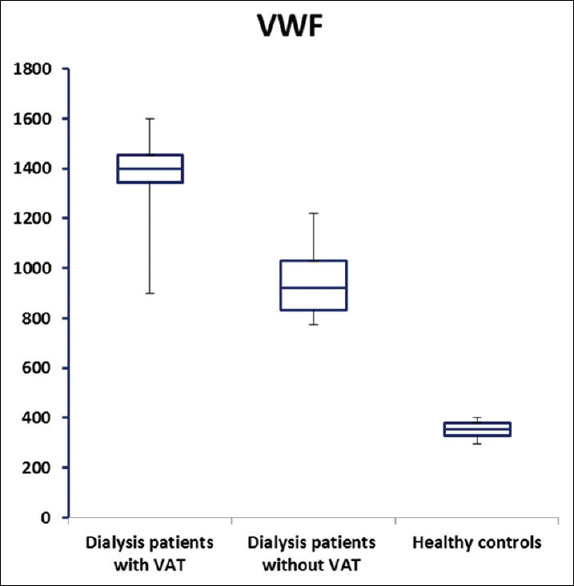
- von Willebrand factor (ng/ml) plasma levels in dialysis patients with, without vascular access thrombosis and healthy control group
Discussion
Hemodialysis as renal replacement therapy is associated with bleeding and thrombotic complications resulting from platelet dysfunction and alterations in both fibrinolytic and coagulation factors.[14] It seems that this hypercoagulation in HD patients is a synergism between genetic (e.g., prothrombin gene mutation, Factor V Leiden, protein C, and protein S) and acquired risk factors.[15]
HD patients experience 20%–25% of their hospitalizations due to vascular access problems, of which thrombosis constitutes 85% of these problems.[16] Furthermore, vascular access dysfunction represents a noteworthy monetary burden for HD patients, in a few appraisals, 10% of the Medicare spending for ESRD patients.[17] ADAMTS13 and VWF had been considered as biomarkers of endothelial dysfunction and kidney disease had been associated with decreased activity of ADAMTS13.[1718]
In the present study, there was a significant statistical increase of plasma VWF levels in HD patients when compared to healthy controls while a significant decrease was observed in ADAMTS13 plasma levels in HD patients than in the control group. These results are aligned with the study by Rios et al., 2012,[1] who demonstrated that, chronic activation of platelet and endothelial cells in HD could continuously increase the VWF levels. Furthermore, higher VWF levels could be result from inflammatory cytokines which stimulate UL-VWF secretion from the endothelium in HD patients.[4]
In addition, Taniguchi et al., 2010[19] and Shen et al., 2012[20] found that increased plasma levels of VWF and reduced ADAMTS13 activity in patients with CKD compared with the control group. Another study by Malyszko et al., 2001[21] illustrated that, endothelial damage in HD patients, caused by uremic state, hyperlipidemia, inflammatory cytokines, or hypertension increased VWF levels. HD patients also experienced higher expression of endothelial VWF, thrombomodulin, and tissue factor.
Ono et al., 2006[22] and Cohen-Hagai et al., 2017[10] demonstrated that higher concentrations of VWF were associated with increased breakdown of ADAMTS13. This breakdown is performed by either proteases present in plasma such as thrombin or secreted by granulocyte elastases. Higher thrombin levels might increase ADAMTS13 turnover in hypercoagulable states. ADAMTS13 activity also was decreased in other conditions complicated by hypercoagulable states such as CKD, chronic liver disease, cardiovascular disease, and disseminated intravascular coagulation.[22232425]
Furthermore, there was increased plasma VWF and decreased ADAMTS13 levels in preeclampsia[5] and diabetic nephropathy, with a negative correlation with estimated glomerular filtration rate.[25] ADAMTS13 malfunction either due to its deficiency and/or antibodies may support the event of thrombosis in small vessels because UL-VWF plasma levels were high. Higher plasma levels of VWF multimers due to absence or low ADAMTS13 activity as seen in hemolytic uremic syndrome and thrombotic thrombocytopenic purpura, had thrombotic tendency.[8]
This study revealed that HD patients with VAT showed significantly higher VWF levels and significantly lower ADAMTS13 levels than those without VAT [Table 2]. These higher VWF levels and lower ADAMTS13 activity may be incriminated in vascular access dysfunction. It was found that higher UL-VWF levels may be linked with widespread obstruction of renal arterioles.[26] Small isomers of VWF did not adhere to platelets after splitting of UL-VWF by ADAMTS13. Although the UL-VWF form aggregates with platelets and lead to occlusion of microcirculation.[4]
Danis et al., 2009[27] found that in HD patients, ADAMTS13, and VWF imbalance is a common component of a hypercoagulability state which, in addition to other genetic and acquired factors, could trigger the thrombotic events in these patients. Rios et al., 2011[28] stated that prothrombin gene mutation (G20210A) was associated to the development of VAT in HD patients. The balance between decreased ADAMTS13 activity and increased VWF observed in dialysis patients may contribute to the prothrombotic state and accelerated atherosclerosis.[29]
On contrary to our results, Rios et al., 2012[1] reported that the antibody capture ELISA test used to detect VWF recognizes mainly ULVWF, and there was no significant differences in levels of VWF between, patients with and without VAT, suggesting that HD initiate a hypercoagulability state, regardless of the development of VAT.
Study limitations
It was a cross-sectional study and did not provide causal relationship. The sample size is small. Further prospective studies with large number of patients are thus needed to confirm our results.
Conclusion
The study concluded that plasma VWF and ADAMTS13 might be considered as markers of hypercoagulability state in HD patients as they are associated with VAT occurrence. So, further prospective studies with large number of patients are thus needed to confirm our results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- ADAMTS13 and von Willebrand factor in patients undergoing hemodialysis. J Thromb Thrombolysis. 2012;34:73-8.
- [Google Scholar]
- Hemostatic changes in patients with end stage renal disease undergoing hemodialysis. Clin Chim Acta. 2010;411:135-9.
- [Google Scholar]
- Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1:1335-42.
- [Google Scholar]
- Relapse of thrombotic thrombocytopenic purpura associated with decreased VWF cleaving activity. Am J Med Sci. 2002;323:281-4.
- [Google Scholar]
- ADAMTS13, FVIII, von Willebrand factor, ABO blood group assessment in preeclampsia. Clin Chim Acta. 2011;412:2162-6.
- [Google Scholar]
- VWF excess and ADAMTS13 deficiency: A unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thromb Haemost. 2015;113:708-18.
- [Google Scholar]
- Von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578-84.
- [Google Scholar]
- ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106:1262-7.
- [Google Scholar]
- A novel human metalloprotease synthesized in the liver and secreted into the blood: Possibly, the von Willebrand factor-cleaving protease? J Biochem. 2001;130:475-80.
- [Google Scholar]
- Effect of Vitamin D status on von Willebrand factor and ADAMTS13 in diabetic patients on chronic hemodialysis. Ann Lab Med. 2017;37:155-8.
- [Google Scholar]
- The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med. 1993;329:1001-6.
- [Google Scholar]
- The role of von Willebrand factor as a biomarker of tumor development in hepatitis B virus-associated human hepatocellular carcinoma: A quantitative proteomic based study. J Proteomics. 2014;106:99-112.
- [Google Scholar]
- R&D Systems: Products & Services for Cell Biology. Available from: https://www.rndsystems.com/
- Differences in coagulation in clotting of vascular access in hemodialysis patients. Hemodial Int. 2015;19:323-9.
- [Google Scholar]
- Coagulation and haemodialysis access thrombosis. Nephrol Dial Transplant. 2000;15:1755-60.
- [Google Scholar]
- ADAMTS13 predicts renal and cardiovascular events in type 2 diabetic patients and response to therapy. Diabetes. 2013;62:3599-609.
- [Google Scholar]
- Association between reduced ADAMTS13 and diabetic nephropathy. Thromb Res. 2010;125:e310-6.
- [Google Scholar]
- Von Willebrand factor, ADAMTS13 activity, TNF-α and their relationships in patients with chronic kidney disease. Exp Ther Med. 2012;3:530-4.
- [Google Scholar]
- Comparison of hemostatic disturbances between patients on CAPD and patients on hemodialysis. Perit Dial Int. 2001;21:158-65.
- [Google Scholar]
- Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: Its correlation with development of renal failure. Blood. 2006;107:528-34.
- [Google Scholar]
- Acquired ADAMTS-13 deficiency in pediatric patients with severe sepsis. Haematologica. 2007;92:121-4.
- [Google Scholar]
- Plasma von Willebrand factor, thrombosis, and the endothelium: The first 30 years. Thromb Haemost. 2006;95:49-55.
- [Google Scholar]
- Significance of plasma von Willebrand factor level and von Willebrand factor-cleaving protease activity in patients with chronic renal diseases. Chin Med J (Engl). 2008;121:133-6.
- [Google Scholar]
- Thrombophilias and arteriovenous fistula dysfunction in maintenance hemodialysis. J Thromb Thrombolysis. 2009;27:307-15.
- [Google Scholar]
- Hemodialysis vascular access thrombosis: The role of factor V leiden, prothrombin gene mutation and ABO blood groups. Clin Chim Acta. 2011;412:425-9.
- [Google Scholar]
- Kidney function and multiple hemostatic markers: Cross sectional associations in the multi-ethnic study of atherosclerosis. BMC Nephrol. 2011;12:3.
- [Google Scholar]







