Translate this page into:
Post-CMV Organizing Pneumonia – An Unusual Presentation 10 years after Kidney Transplantation
Address for correspondence: Dr. Lovy Gaur, Max Superspeciality Hospital, W-3, Sector-1, Vaishali, Ghaziabad, Uttar Pradesh, India. E-mail: drlovygaur@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A 45-year-old gentleman underwent kidney transplantation in March 2010. He remained apparently healthy for the next 10 years when he developed anorexia and weight loss. Diagnostic workup revealed cytomegalovirus (CMV) pneumonia. While viremia resolved within 3 weeks of initiation of valganciclovir, he developed progressive breathlessness and hypoxia on exertion. Imaging of thorax revealed central peri-bronchovascular consolidation and fine reticulations with peripheral sparing. Computed tomography (CT)-guided percutaneous lung biopsy revealed organizing intra-alveolar exudates, suggestive of organizing pneumonia, with no evidence of active infection on biopsy as well as bronchoalveolar lavage (BAL) cytology. This atypical pattern of central distribution of opacities is not typical of organizing pneumonia where peripheral subpleural distribution is more common. Patient responded dramatically following escalation of steroids, with complete resolution of infiltrates on follow-up imaging.
Keywords
Bronchiolitis Obliterans with Organizing Pneumonia
cytomegalovirus (CMV) pneumonia
kidney transplant recipient
organizing pneumonia
Introduction
Cytomegalovirus (CMV) is the most common viral pathogen responsible for pneumonia in solid organ transplant recipients. Early diagnosis and directed treatment are the cornerstones of successful management. In this case report, we describe a kidney transplant recipient on minimal immune suppression who developed CMV pneumonia that was treated adequately with antiviral medicines and later evolved into organizing pneumonia complicating the clinical picture. The diagnosis was established on computed tomography (CT)-guided percutaneous needle lung biopsy, and the patient was treated with steroids, which led to complete recovery.
Case Report
A 45-year-old gentleman, known case of end-stage kidney disease, underwent kidney transplant in March 2010. His sister with one haplotype HLA match was the donor. Both the recipient and the donor were seropositive for CMV IgG. He did not receive any induction by lymphocyte-depleting therapy, and maintenance therapy consisted of tacrolimus (2 mg/day targeted to level 5–7 ng/ml), mycophenolate sodium (720 mg/day), and prednisolone (5 mg/day). Baseline creatinine was 1.4 mg/dl. Almost a decade later, he had new-onset proteinuria and rise in creatinine to 2 mg/dl, for which he underwent a graft kidney biopsy in May 2019, which was suggestive of transplant glomerulopathy with 30%–40% interstitial fibrosis and tubular atrophy. Following this, mycophenolate sodium was increased to 360 mg thrice daily, while tacrolimus and prednisolone were continued at the same dose. Target tacrolimus levels were 5–7 ng/ml (achieved levels were 6.2 and 5.8 ng/ml on two occasions, respectively, over the following 8 months). Telmisartan was initiated in addition to fixed-dose combination of amlodipine and atenolol which the patient had already been receiving for blood pressure control.
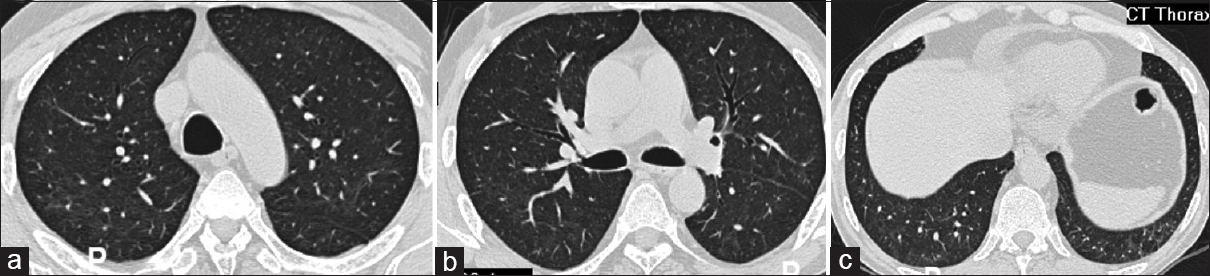
Eight months later (in March 2020), he presented with loss of appetite and loss of approximately 10 kg weight in the preceding 4 months. There was no fever, cough, vomiting, diarrhea, or jaundice. Hematological and biochemical tests (including liver function tests) were unremarkable. Serology was negative for human immunodeficiency virus (HIV) and hepatitis viruses. Imaging studies including chest X ray and ultrasound of abdomen did not reveal any abnormality. In view of persistent symptoms and to rule out any occult infections and malignancies, a CT scan of chest and abdomen was done. CT thorax was remarkable for the presence of diffuse ground-glass opacities (GGO) in both lung fields [Figure 1]. There was no consolidation, nodule formation, or lobar predilection. Minimal subpleural sparing was seen. The overall CT findings were suggestive of viral pneumonia. Oronasal swab for coronavirus disease real-time polymerase chain reaction (COVID-RT PCR) was negative. CMV quantitative RT-PCR of blood revealed 21,128 copies/ml. Mycophenolate sodium was stopped and prednisolone was increased to 10 mg/day. The patient was given oral valgancyclovir for 3 weeks.

- CT thorax shows diffuse areas of ground-glass attenuation throughout bilateral lung parenchyma with sparing of subpleural zones, CT = computed tomography; (a) section of bilateral upper lobes, (b) section of right middle lobe and left lower lobe, (c) bilateral lower lobes with ground-glass attenuation
After 3 weeks, his CMV-PCR became negative, but he developed shortness of breath and hypoxia on exertion. There was no fever/cough or sputum production. On presentation, blood pressure was 140/90 mmHg, pulse rate 98/min, and respiratory rate 28/min. Examination revealed diffuse inspiratory crepitations. A CT scan was done again, which revealed that the previously involved areas of bronchocentric GGO were replaced by areas of early central peri-bronchovascular consolidation and fine reticulations [Figure 2]. Minimal bronchiolectasis was seen, but there was no lung scarring or architectural distortion. Bronchoscopy was unremarkable, and bronchoalveolar lavage (BAL) did not reveal any organism on Gram stain, Ziehl–Neelsen, wet mount KOH, silver-methenamine, and immunofluorescence for Pneumocystis jirovecii. Respiratory panel PCR for bacteria and viruses was also negative. Transbronchial lung biopsy was refused due to technical reasons. A CT-guided, percutaneous needle lung biopsy was performed from the densest patch of GGO/consolidation in the right upper lobe, which revealed alveolar spaces filled with loose fibroblasts and macrophages admixed with myxoid ground substance (Masson bodies) [Figure 3]. Lung architecture was relatively preserved and most alveoli lacked significant fibrosis. There were no demonstrable granulomas, giant cells, viral cytopathic changes, or Pneumocystis spp. Based on these features, a diagnosis of organizing pneumonia, likely post-CMV, was made and the patient was started on prednisolone 40 mg/day. Over the following 4 weeks, patient improved symptomatically. Prednisolone was gradually tapered over the next 3 months to 5 mg/day and tacrolimus was continued at the same dose targeted to maintain levels between 5 and 7 ng/ml. Valganciclovir was continued for another 3 months. Follow-up chest radiographs and a repeat CT scan showed no residual sequel [Figure 4].

- CT thorax shows coalescent peribronchial nodular opacities with interspersed areas of ground-glass attenuation. (a), (b) and (c) images show different axial sections of lungs with diffuse peribronchial nodular opacities, (d) coronal section of lungs highlighting diffuse involvement of the lungs bilaterally, (e) sagittal section of the lung, (f) Percutaneous CT guided lung biopsy
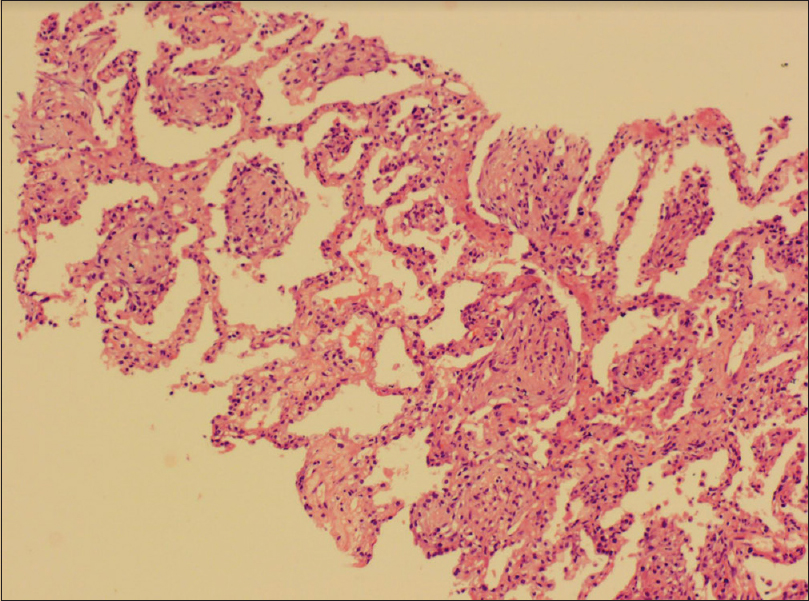
- Lung tissue shows alveolar spaces filled with loose fibroblasts and macrophages along with myxoid ground substance
- CT thorax shows complete resolution of nodules and ground-glass opacities. (a) Axial section of CT lung showing bilateral upper lobes, (b) section highlighting right middle and left lobes and (c) bilateral lower zone of lungs
Discussion
CMV is one of the common infections seen in patients receiving solid organ transplants. Without prophylaxis, CMV disease develops in 10%–60% of kidney transplant recipients, with the risk being the highest in D+/R˗ CMV serostatus at the time of transplantation and negligible with D˗/R˗ transplants.[1] Net status of immunosuppression also plays a role in endogenous reactivation of CMV disease. This, in turn, is governed by a complex interplay of recipient factors (age, gender, genetic polymorphism, HLA mismatch), immunosuppressive agents (antithymocyte globulin, drug dosage), graft function, concurrent infections, other systemic comorbidities, and malnutrition.[12]
Oral valganciclovir is an effective therapy for CMV disease.[3] Our patient too responded well to treatment with valganciclovir, and viral copies were undetectable by 3 weeks. However, viremia clearance was followed by organizing pneumonia secondary to alveolar injury due to CMV.
Organizing pneumonia is a distinct clinicopathological entity presenting with generalized malaise, hypoxia, and restrictive lung disease. Imaging studies usually reveal consolidation and GGO, which are subpleural and bilateral.[45] Histologically, it is characterized by patchy filling of alveoli by loose plugs of granulation tissue (Masson bodies). The predominant feature is alveolar inflammation and filling process than bronchiolitis.[6] Over time, changes may become more organized and diffuse, but lung architecture is well preserved.
Organizing pneumonia may occur secondary to infections, drugs, and collagen vascular disease or may be idiopathic. In the posttransplant population, it has been well reported in recipients of lung transplants[57] and in allogenic hematopoietic stem cell transplants.[8] Relatively fewer cases have been reported in kidney transplant recipients [Table 1]. The first case of organizing pneumonia after kidney transplantation was described by Verberckmoes et al.,[9] wherein no etiology was identified. Two similar cases of cryptogenic organizing pneumonia were also reported by Kute et al.[10] and Hasni et al.[11] In other cases, sirolimus was the probable agent implicated in the occurrence of organizing pneumonia.[12131415] Recovery in these cases required discontinuation of sirolimus and escalation of steroid doses.
| Age | Sex | Background illness | Etiology of organizing pneumonia | Treatment | Outcome | |
|---|---|---|---|---|---|---|
| Verberckmoes et al.[9] | 26 | M | KTR (4 months) | Idiopathic | Methylprednisolone escalated from 10 to 35 mg/day | Recovered |
| Jacques et al.[16] | 59 | F | KTR (3 months), SLE | CMV | Steroids 1 mg/kg/day | Recovered |
| Hasni et al.[11] | 54 | M | Second transplant (2 years) | Idiopathic | Not specified | Not known |
| Cunha[17] | 77 | M | HTN, DM, CHF, KTR (4 years) | HSV (on BAL) ?Tac | Tac discontinued; acyclovir continued | Died due to worsening of CHF |
| Kute et al.[10] | 42 | M | KTR (2 years) | Idiopathic | Methylprednisolone 250 mg IV × 3 days, prednisolone 40 mg/day for 4 weeks | Recurrence on steroid taper, recovered eventually on re-escalation |
| Kodati et al.[18] | 36 | M | KTR (6 months) | Pneumocystis jirovecii (on BAL) | Asymptomatic. prednisolone continued at the same dose (10 mg/day) | Recovered |
BAL=bronchoalveolar lavage, CHF=congestive heart failure, CMV=cytomegalovirus, DM=diabetes mellitus, HSV=herpes simplex virus, HTN=hypertension, IV=intravenous, KTR=kidney transplant recipient, SLE=systemic lupus erythematosus, Tac=tacrolimus
A similar case of organizing pneumonia post-CMV was described by Jacques et al.[16] The patient, however, developed early CMV disease soon after withdrawal of valacyclovir prophylaxis (15 days). A week into intravenous ganciclovir treatment, the patient developed organizing pneumonia. The patient responded well to steroid escalation.
There are two very intriguing features in the course of the present patient's illness.
Overall, the patient was at a low to intermediate risk of developing CMV disease based on the haplo-match donor, absence of use of any thymocyte-depleting agent and seropositive status (D+/R+) with respect to CMV IgG, and 10 years of posttransplant course on stable immunosuppression. Secondly, organizing pneumonia complicated the course of the patient who had been recovering well from CMV infection. While the subpleural location of opacities is more typical of organizing pneumonia, the patient had predominantly central opacities. Histopathology helped in clinching the unusual diagnosis and ruling out other differentials, which is of prime importance since the patient responded well to treatment without any long-term sequelae.
Declaration of patient consent
The authors certify that they have obtained patient's consent. The patient understands that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Post-transplant infections: An ounce of prevention. Indian J Nephrol. 2010;20:171-8.
- [Google Scholar]
- Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant. 2009;9:1205-13.
- [Google Scholar]
- Cryptogenic and secondary organizing pneumonia: Clinical presentation, radiographic findings, treatment response, and prognosis. Chest. 2011;139:893-900.
- [Google Scholar]
- Bronchiolitis obliterans organizing pneumonia (BOOP) in lung transplant recipients. Chest. 1996;110:1150-4.
- [Google Scholar]
- Cytomegalovirus pneumonitis complicated by a central peribronchial pattern of organising pneumonia. Respir Med Case Rep. 2017;20:184-7.
- [Google Scholar]
- BOOP (Bronchiolitis obliterans organizing pneumonia) after renal transplantation. Nephrol Dial Transplant. 1996;11:1862-3.
- [Google Scholar]
- Bronchiolitis obliterans organizing pneumonia after renal transplantation. Int Uro Nephrol. 2013;45:1517-21.
- [Google Scholar]
- Bronchiolitis obliterans organizing pneumonia in renal transplant patients. Dial Transplant. 2010;39:449-51.
- [Google Scholar]
- Sirolimus-induced pneumonitis complicated by pentamidine-induced phospholipidosis in a renal transplant recipient: A case report. Transplant Proc. 2011;43:2792-7.
- [Google Scholar]
- An unusual presentation of sirolimus associated cough in a renal transplant recipient. Transplant Proc. 2007;39:3463-4.
- [Google Scholar]
- Pneumonitis associated with sirolimus: Clinical characteristics, risk factors and outcome--A single-centre experience and review of the literature. Nephrol Dial Transplant. 2007;22:3631-7.
- [Google Scholar]
- Sirolimus-induced bronchiolitis obliterans organizing pneumonia in a kidney transplant recipient; a case report and review of literature. J Nephropathol. 2014;3:109-13.
- [Google Scholar]
- An unusual pulmonary complication of cytomegalovirus infection in a renal transplant recipient. NDT Plus. 2008;4:236-8.
- [Google Scholar]
- Renal transplant with bronchiolitis obliterans organizing pneumonia (BOOP) attributable to tacrolimus and herpes simplex virus (HSV) pneumonia. Heart Lung. 2012;41:310-5.
- [Google Scholar]
- Organizing pneumonia secondary to Pneumocystis jirovecii infection in a kidney transplant recipient: Case report and review of literature. Lung India. 2020;37:441-4.
- [Google Scholar]







