Translate this page into:
Post-Diarrheal Acute Kidney Injury During an Epidemic in Monsoon – A Retrospective Study from a Tertiary Care Hospital
Corresponding author: Poongodi Annadurai, Department of Nephrology, Stanley Medical College and Hospital, Old Jail Road, Chennai, Tamil Nadu, India. E-mail: poongodr1981@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Haridas N, Thirumavalavan S, Fernando ME, Vellaisamy M, Annadurai P, Srinivasaprasad ND, et al. Post-Diarrheal Acute Kidney Injury During an Epidemic in Monsoon – A Retrospective Study from a Tertiary Care Hospital. Indian J Nephrol. 2024;34:338-43. doi: 10.25259/ijn_285_23
Abstract
Background:
Acute kidney injury (AKI) is a severe complication of acute diarrheal diseases; however, there is limited data on post-diarrheal AKI (PD-AKI) epidemiology and outcomes. This study aimed to investigate the clinicodemographic profile and outcomes of PD-AKI in our hospital.
Materials and Methods:
We retrospectively analyzed data from 93 patients admitted with PD-AKI during a diarrheal illness epidemic. Patients were stratified based on the Kidney Disease: Improving Global Outcomes (KDIGO) AKI stage and quick Sequential Organ Failure Assessment (qSOFA) score. Clinicodemographic data and outcomes were recorded and analyzed.
Results:
The mean age of the patients was 45.7 ± 11.9 years, with a majority being men (n = 55, 59%). All patients presented with watery diarrhea, 85% (n = 79) had vomiting, and 66% (n = 61) presented in shock. At presentation, 59% were oliguric, while 32% were anuric. KDIGO stage 3 AKI was observed in 71% (n = 66) of patients. Dialytic support was required in 29% (n = 27) of cases. The mortality rate was 6.5% (n = 6), mostly due to refractory shock, while the remaining patients recovered. Risk factor analysis demonstrated a higher qSOFA score, and peak serum creatinine levels were associated with an increased likelihood of requiring renal replacement therapy and delayed renal recovery.
Conclusion:
This study provides valuable insights into the clinicodemographic characteristics and outcomes of PD-AKI. The high prevalence of severe AKI emphasizes the importance of early recognition and appropriate management strategies for these patients.
Keywords
Acute diarrheal disease
acute kidney injury
epidemic
vibrio
Introduction
Acute kidney injury (AKI) is a syndrome resulting from a rapid loss of kidney function as quantified by a reduction in the glomerular filtration rate (GFR) leading to the retention of nitrogenous waste products and is often the most common cause for a nephrology consultation in inpatient medical practice. AKI is defined as a functional or structural kidney abnormality that manifests with an increase in serum creatinine (Cr) of 0.3 mg/dL or greater within 48 hours, an increase in serum Cr of 1.5 or greater times baseline within 7 days, or a urine volume less than 0.5 mL/kg/h for 6 hours.1 AKI is also associated with high rates of morbidity and mortality. In critically ill patients with severe ATN requiring dialytic support, hospital mortality rates are in the range of 40%–70%.2 AKI, previously considered a single disease, has now evolved into a complex multifactorial syndrome with varying pathophysiology and prognosis. Before 2004, more than 50 definitions of AKI (previously called “acute renal failure”) were used, and the reported incidences, prevalence, and outcomes were quite variable.3 With the usage of more standardized definitions and staging of AKI after the initial Risk, Injury, Failure, Loss, and End-stage kidney disease criteria4 of 2004, followed by the Acute Kidney Injury Network4 classification of 2007 and the newer Kidney Disease: Improving Global Outcomes (KDIGO) staging1 in 2012, epidemiological studies have shown a high prevalence of AKI worldwide along with its high rate of both short- and long-term complications. Recently, the impact of AKI on an increased risk of progression to chronic kidney disease (CKD) and end-stage renal disease requiring renal replacement therapies is gaining increased attention.5 Renal recovery is also found to be significantly delayed in patients with severe AKI requiring renal replacement therapy compared to those managed conservatively.6 Considering the importance of early recognition and treatment of AKI, the International Society of Nephrology launched the 0by25 Initiative in 2013, which aims to eliminate preventable deaths from AKI worldwide by 2025.7
Gastroenteritis, characterized by inflammation of the stomach, small intestine, or large intestine, manifests as a combination of symptoms, including abdominal pain, nausea, vomiting, and diarrhea. Acute gastroenteritis typically resolves within 14 days. Acute gastroenteritis and acute diarrheal illness can be caused by viruses, bacteria, protozoa, and parasites. Enteric pathogens are frequently transmitted through contaminated food or water, and some pathogens with low infectious doses may also spread from person to person. While these pathogens often lead to localized outbreaks, they also hold the potential to trigger pandemics and are among the important causes of morbidity and mortality, especially in populations with limited access to safe drinking water and adequate sanitation.
Acute gastroenteritis and acute diarrheal diseases are frequently complicated by AKI, often preventable if intervened early. Inadequate or delayed restoration of diarrheal losses results in a very high incidence of AKI, commonly either prerenal or acute tubular injury, and less commonly acute tubulointerstitial nephritis.8 Diarrheal illness is also the most common etiology for hemolytic uremic syndrome.9 Infection-related glomerulonephritis and acute cortical necrosis following acute gastroenteritis have also been reported.10,11 Diarrheal illness is a major reason for hospitalization in tropical countries, and it can lead to various complications secondary to volume depletion, including shock, AKI, electrolyte disturbances, and metabolic acidosis. However, the data on post-diarrheal acute kidney injury (PD-AKI) is sparse as it is often not reported. Seasonal epidemics of diarrheal illness are common, especially around the monsoon season. This retrospective study aimed to analyze the clinical profile of patients with PD-AKI during an epidemic in the monsoon of 2021.
This study analyzed the burden of PD-AKI, the clinical profile of patients, and the outcomes and prognosis of PD-AKI of the patients admitted during an epidemic during the monsoon of 2021 at our hospital.
Materials and Methods
It is a retrospective observational study involving patients admitted to our hospital with acute diarrheal illness between October and December 2021. The study was approved by the institutional ethics committee at Stanley Medical College, Chennai, number 20220203, dated 1st February 2022.
According to the hospital records of patients admitted during the study period, 923 adults were admitted with acute diarrheal illness; 10.07% (n = 93) of the patients were found to have AKI and were included in the study. AKI was stratified according to the 2012 KDIGO staging1 as follows: stage 1 (Cr increase ≥ 0.3 mg/dL within the past 48 hours or an increase of 1.5–1.9 times the baseline or a urine output <0.5 mL/kg/h for 6–12 hours), stage 2 (Cr increase of 2.0–2.9 times the baseline value or a urine output of <0.5 mL/kg/h for ≥12 hours), and stage 3 (Cr increase of 3 times the baseline value or serum Cr ≥ 4 mg/dL or RRT initiation or a urine output of <0.3 mL/kg/h for ≥24 hours or anuria for ≥12 hours). Patients above 70 years and those patients who had a prior history of CKD defined by estimated DFR ([eGFR] < 60 mL/min/1.73 m2), prior renal transplant, or patients having contracted kidneys on abdominal sonography were excluded from the study. Patients were also stratified according to the quick Sequential Organ Failure Assessment (qSOFA) score.12 Data on demographic characteristics, clinical features, comorbidities, biochemical parameters, stool microbiology, the treatment provided (including renal replacement therapy), and outcomes were retrieved and recorded using a standardized data form and analyzed. The study flow is summarized in Figure 1.
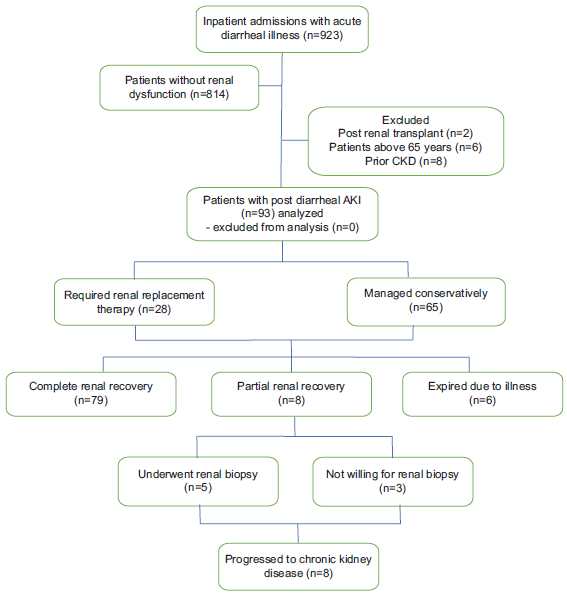
- Study flow. AKI: Acute kidney injury, CKD: chronic kidney disease
Statistical methods
To describe the data, frequency analysis and percentage analysis were used for categorical variables. For continuous variables, mean with standard deviation (SD) was used for normal data, and median with interquartile range (IQR) was used for non-normal data. Patients were stratified into three groups based on KDIGO staging.1 The association of all the categorical between the groups was assessed using the chi-square test. The comparison of the continuous variables between the groups was assessed using the Mann–Whitney U test. The risk factors with P < 0.1 for the primary outcome (need for renal replacement therapy) identified with univariate analysis using the Chi-square test were further assessed using binary regression analysis. Statistical significance was considered at P < 0.05; odds ratios with a 95% confidence interval were also calculated. Statistical analysis was done using IBM SPSS statistics software version 19.0.
Results
A total of 923 adult patients with diarrheal illness were admitted during the epidemic period, out of which 10.07% (n = 93) were diagnosed with PD-AKI and were included in the study. During the study period of 3 months, of the total 313 AKI patients treated in our hospital, 29% had PD-AKI. Demographic, clinical, and hospital characteristics of patients enrolled patients are summarized in Tables 1 and 2. The mean age of the patients was 45.7 ± 11.9 years, with a majority being male (n = 55, 59%). Furthermore, 63% (n = 59) of the patients resided in urban areas, while 37% (n = 34) were from rural areas. Comorbidities were observed in the patient population, with 28% (n = 23) having type 2 diabetes, 30% (n = 28) being hypertensive, and 24% (n = 22) having a history of alcohol consumption. All patients presented with diarrhea, with 85% (n = 79) experiencing vomiting and 66% (n = 61) presenting in a state of shock. At admission, 63.4% (n = 59) of patients were oliguric, 32.3% (n = 30) were anuric, and 4.3% (n = 4) did not report reduced urine output. Based on the qSOFA scoring system, 54.8% (n = 51) had a score of 2, 24.7% (n = 23) had a score of 1, and 18.3% (n = 17) had a score of 3. Vasopressor support was required in 22.6% (n = 21) of patients due to refractory hypotension, while 5.4% (n = 5) required mechanical ventilatory support.
| Parameter | Total (n = 93) | KDIGO 1 (n = 1) | KDIGO 2 (n = 26) | KDIGO 3 (n = 66) | P value |
|---|---|---|---|---|---|
| Age in years (Mean±SD) | 45.7 ± 11.9 | 38 | 43 ± 13.3 | 46.9 ± 11.4 | 0.28 |
| Gender | |||||
| Male (n, %) | 55 (59.1) | 1 (100) | 21 (80.8) | 33 (50) | |
| Female (n, %) | 38 (41) | 0 | 5 (19.2) | 33 (50) | |
| Residence | |||||
| Urban (n, %) | 59 (63.4) | 0 | 14 (53.8) | 45 (68.2) | 0.16 |
| Rural (n, %) | 34 (36.6) | 1 (100) | 12 (46.2) | 21 (31.8) | |
| Comorbidities | |||||
| Diabetes mellitus | 23 (24.7) | 0 | 8 (30.8) | 15 (22.7) | 0.6 |
| Hypertension | 28 (30.1) | 1 (100) | 6 (23.1) | 21 (31.8) | 0.2 |
| Coronary artery disease | 2 (2.2) | 0 | 2 (7.7) | 0 | 0.07 |
| Alcoholism | 22 (23.7) | 1 (100) | 3 (11.5) | 18 (27.3) | 0.05 |
| Stroke | 2 (2.2) | 0 | 0 | 2 (3.0) | 0.66 |
| Obesity | 4 (4.3) | 0 | 1 (3.8) | 3 (4.5) | 0.97 |
| qSOFA score | 1.89 ± 0.7 | 1 | 1.6 ± 0.75 | 2±0.65 | 0.015 |
| Vasopressor use | 21 (22.6) | 0 | 4 (15.4) | 17 (25.8) | 0.48 |
| Need for RRT n (%) | 27 (29.0) | 27 (40.9) | |||
| Need for ventilatory support | 5 (5.4) | 0 | 0 | 5 (7.6) | 0.34 |
RRT: renal replacement therapy; qSOFA: quick sequential organ failure assessment; KDIGO: Kidney disease improving global outcomes.
| Clinical Features | |
|---|---|
| Duration of diarrhea in days (mean ± SD) | 2.4 ± 1.1 |
| Hypotension (SBP < 90/DBP < 60) n (%) | 61 (65.6) |
| Urine Output n (%) | |
| Oliguric | 59 (63.4) |
| Anuric | 30 (32.3) |
| Non-oliguric | 4 (4.3) |
| Fever (%) | 29 (31.2) |
| Lab Parameters | |
|---|---|
| Peak creatinine mg/dL, (mean ± SD) | 5.7 ± 3 |
| Hyponatremia (Serum Na < 135) n (%) | 43 (46%) |
| Hypokalemia (Serum K < 3.5) n (%) | 48 (51.6) |
| Hypernatremia (Serum Na > 145) n (%) | 10 (10.7) |
| Hyperkalemia (Serum K > 5.5) n (%) | 4 (4.3) |
| Metabolic acidosis (Serum HCO3 < 16) n (%) | 19 (20.4) |
SBP: systolic blood pressure; DBP: diastolic blood pressure; Na: sodium; K: potassium; HCO3: bicarbonate
Of the 923 patients admitted with diarrheal illness, 78% (n = 78) sought medical care within 3 days after the onset of loose stools. The mean peak serum Cr level observed in the patient cohort was 5.7 ± 3 mg/dL. In addition, the mean potassium level was 3.67 ± 0.9 mmol/L, with 51% (n = 48) of patients presenting with hypokalemia (serum potassium < 3.5 mmol/L) upon admission. Among the patients, 71% (n = 66) were classified as having KDIGO stage 3 AKI. Notably, the severity of AKI was significantly greater in alcoholics [27.3% (n = 18) were in KDIGO stage 3, P = 0.05]. Stool microbiology (hanging drop and culture) was positive for Vibrio in 8.6% (n = 8) patients, while it was negative for enteric pathogens in all others. Furthermore, 29% (n = 27) of the patients required dialytic support. Dialytic support was required in 29% (n = 27) of the patients for standard indications. Specifically, six patients initially received acute intermittent peritoneal dialysis, with one patient later transitioning to intermittent hemodialysis. In addition, 21 patients received intermittent hemodialysis as the modality for renal replacement therapy. Metabolic acidosis was the most common indication for dialysis noted in our patients (55.6% of patients who received RRT, n = 15), followed by uremia (37%, n = 10). Moreover, 6.5% (n = 6) of patients succumbed to the illness due to refractory shock, while 87 patients recovered with the treatment given. At 1 month of follow-up, 8.6% (n = 8) patients had only a partial recovery of renal function but were dialysis independent. Five of these patients with partial renal recovery underwent percutaneous renal biopsy, while the remaining patients declined the procedure. The biopsy results indicated acute tubular injury without any significant chronicity in four patients, and one patient had features of infection-related glomerulonephritis. These eight patients had persistent renal dysfunction at 3 months of follow-up and were diagnosed to have progressed to CKD. All other patients (84.9%, n = 79) had complete recovery of renal function.
Univariate analysis revealed that higher qSOFA score, higher peak Cr during hospitalization, and the need for mechanical ventilatory support were significantly associated with the requirement for dialysis. Subsequent multivariate analysis confirmed that higher peak serum Cr (P = 0.00, OR 1.55) and the need for mechanical ventilatory support (P = 0.016, OR 19.9) were independent predictors of dialysis requirement. Similarly, univariate analysis of factors associated with delayed renal recovery (≥21 days) identified higher qSOFA score, higher peak serum Cr during admission, and the requirement for renal replacement therapy as significant factors. The multivariate analysis determined that the requirement for renal replacement therapy was an independent predictor of delayed renal recovery (P = 0.004, OR 17). Due to the limited number of fatalities (n = 6), our study lacked sufficient statistical power to comprehensively assess the factors contributing to mortality. Nevertheless, we observed a higher mortality rate among patients of higher age. The statistical analysis of risk factors associated with dialysis requirement and delayed renal recovery, and the patient outcomes are summarized in Tables 3 and 4.
| Dialysis requirement | Delayed renal recovery (≥21 days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
| P | P | OR | Confidence interval | P | P | OR | Confidence interval | |||
| Upper | Lower | Upper | lower | |||||||
| Age | 0.65 | 0.53 | ||||||||
| Sex | 0.171 | 0.85 | ||||||||
| Diabetes mellitus | 0.72 | 0.62 | ||||||||
| Hypertension | 0.665 | 0.98 | ||||||||
| Duration of diarrhea | 0.62 | 0.99 | ||||||||
| Alcohol abuse | 0.46 | 0.76 | ||||||||
| Shock on admission | 0.54 | 0.376 | ||||||||
| Lonotrope requirement | 0.62 | 0.258 | ||||||||
| qSOFA score | 0.03 | 0.278 | 1.62 | 0.678 | 3.87 | 0.049 | 0.19 | 1.9 | 0.72 | 5.4 |
| Peak BUN | 0.22 | 0.099 | 0.5 | 1.0 | 0.99 | 1. | ||||
| Peak serum creatinine | 0.00 | 0.00 | 1.55 | 1.25 | 1.93 | 0.004 | 0.574 | 1.115 | 0.764 | 1.63 |
| RRT requirement | 0.00 | 0.004 | 17.23 | 2.57 | 115.4 | |||||
| Mechanical Ventilator support | 0.034 | 0.016 | 19.9 | 1.75 | 225.8 | |||||
OR - odds ratio, BUN - blood urea nitrogen, qSOFA - quick sequential organ failure assessment, RRT: renal replacement therapy
| Total | KDIGO 1 | KDIGO 2 | KDIGO 3 (Non-Dialysis required) 39 | KDIGO 3 (Dialysis required) N 27 | P value | |
|---|---|---|---|---|---|---|
| Renal outcome | ||||||
| Complete recovery | 79 (85) | 1 (100) | 26 (100) | 34 | 18 | 0.03 |
| Partial recovery | 8 (8.6) | 0 | 0 | 4 | 4 | |
| No recovery | 0 | 0 | 0 | |||
| Death | 6 (6.5) | 0 | 0 | 1 | 5 | |
| Time to renal recovery (mean±SD) | 12.4±7.8 | 4 | 5.5±1.4 | 12.5±6.6 | 20.7±6.2 | 0.00 |
| General outcomes | ||||||
| Survived | 87 (93.5) | 1 (100) | 26 (100) | 38 | 22 | 0.27 |
| Expired | 6 (6.5) | 0 | 0 | 1 | 5 |
KDIGO - kidney disease improving global outcomes
Discussion
Acute gastroenteritis and acute diarrheal disease are one of the most common illnesses requiring hospitalization globally.13 Volume depletion leading to hypotension and sepsis are the common causes of AKI in such patients, which is more common in patients with delayed presentation and patients with comorbidities such as diabetes mellitus, systemic hypertension, and coronary artery disease. Severe diarrheal losses can also lead to electrolyte imbalances, metabolic acidosis secondary to bicarbonate loss in stool, and added lactic acidosis due to circulatory collapse. Delayed presentation, late referral to nephrology care, and suboptimal fluid replacement were found to be associated with the increased requirement of dialytic therapy in such patients.14,15 A study conducted by Carpenter CCJ et al.16 showed that rapid and effective extracellular fluid volume restoration within 4 hours can prevent AKI in diarrheal illness. Similar results were found by Prakash et al.,17 wherein it was noted that volume depletion was the most common precipitating factor for AKI. Muthusethupathi et al.18 observed that metabolic acidosis in PD-AKI was a poor prognostic factor and that renal replacement therapy in the form of either hemodialysis or peritoneal dialysis is a valuable therapeutic intervention. Jayakumar et al.19 noted that among the medical causes of AKI, PD-AKI was the most common etiology, responsible for 28.6% of the cases. A more recent study by Vairakkani et al.20 from our center reported a lower incidence of PD-AKI, which accounted for only 5% of the total AKI burden. However, our study, which was done during an epidemic of diarrheal illness, reported a much higher incidence of PD-AKI, which was also the most common cause of AKI in our center during the study period, responsible for 29% of total AKI cases.
In our study, more than half of the patients admitted with diarrheal illness had severe AKI (KDIGO stage 3), with 29% (n = 27) of them requiring renal replacement therapy. Most of our patients had severe watery diarrhea and vomiting, presented with volume depletion, and were clinically suspected to have acute tubular injury secondary to volume depletion, hypotension, and sepsis. All patients received empirical parenteral antibiotics and boluses of isotonic crystalloids in the emergency department. Patients with persistent hypotension despite initial fluid resuscitation were initiated on vasopressor support. None of the patients had clinical or laboratory features suggestive of hemolytic-uremic syndrome.
We noted a significant association between the severity of PD-AKI and alcohol use disorder. This association may be attributed to the diuretic effect of alcohol, leading to impaired renal concentrating capacity, which makes them vulnerable to AKI during hypovolemia. Furthermore, it is worth noting that alcoholics may also experience episodes of diarrhea, which could be a result of consuming contaminated non-vegetarian accompaniments along with alcohol. These findings emphasize the importance of recognizing the potential risks of alcohol use in relation to renal health.
Hypokalemia due to diarrheal losses was noted in most of our patients (51.6%, n = 48), while significant dysnatremia was uncommon. However, we did not assess the Fractional excretion of sodium (FeNa) or urine sodium levels (UNa) in our study as many patients had already received intravenous fluids and diuretics at local hospitals before being referred to our center. Stool microbiology analysis using hanging drop and culture techniques identified Vibrio in eight patients, while no other enteropathogenic organisms were isolated, likely due to the administration of broad-spectrum antibiotics soon after admission.
Metabolic acidosis was the most common indication for renal replacement therapy among our patients, followed by uremia and fluid overload. Hyperkalemia was infrequently encountered, likely due to potassium depletion from diarrheal losses. Only one patient had significant hyperkalemia, prompting the initiation of dialysis. Among the patients in the study cohort, 27 received dialytic support for standard indications. Acute intermittent peritoneal dialysis was offered to six patients due to severe hypotension, with one patient later transitioning to intermittent hemodialysis. In addition, 21 patients received intermittent hemodialysis. At the 1-month follow-up, all surviving patients were independent of dialysis. However, eight patients experienced only partial recovery of renal function. Among these eight patients, five underwent renal biopsy, which revealed features of acute tubular injury without significant chronicity in four patients and infection-related glomerulonephritis in one patient. The remaining patients with partial renal recovery declined renal biopsy. All eight patients with partial renal recovery continued to have persistent abnormal renal function at the end of the 3-month follow-up period, leading to a diagnosis of CKD.
As the number of patients who succumbed to the illness was only 6.4% (n = 6), our study lacked sufficient statistical power to assess the factors contributing to mortality risk. However, we observed that all patients who required mechanical ventilatory support eventually succumbed to the illness. This highlights the importance of respiratory compromise as a significant predictor of mortality in this patient population. We also noted that mortality was higher in older patients. In addition, our study highlighted the relatively rare occurrence of infection-related glomerulonephritis following acute diarrheal illness. This highlights the importance of considering alternative etiologies when evaluating renal dysfunction in the setting of diarrheal illness as it may not always be attributed solely to acute tubular injury or volume depletion.
Our study was subject to some limitations that should be considered when interpreting the results. First, the relatively small number of patients who succumbed to the illness limited our statistical power to detect significant associations concerning mortality. In addition, the lack of FeNa and UNa measurements limited our ability to assess renal tubular function and sodium handling in these patients. Furthermore, the use of broad-spectrum antibiotics early in the course of illness may have affected the isolation of enteropathogenic organisms in stool cultures, potentially underestimating their prevalence. Another limitation of our study was the absence of stool assessment for viral pathogens. Future studies should consider collecting stool samples before the initiation of antibiotic treatment and enteropathogenic viral testing to enhance the accuracy of microbiological investigations.
Conclusion
Epidemics of acute diarrheal illness and its complications can pose a significant burden on the healthcare system of a state. This study provides insights into the clinicodemographic profile and outcomes of PD-AKI, highlighting the importance of early recognition and management. Contact precautions, public health education, and providing access to safe drinking water are necessary goals in decreasing the incidence of diarrheal illness in the community, especially during monsoon.
Conflicts of interest
There are no conflicts of interest.
References
- KDIGO clinical practice guideline for acute kidney injury. Kidney Inter Suppl. 2012;2:1-138.
- [Google Scholar]
- Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-70.
- [CrossRef] [PubMed] [Google Scholar]
- The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin Kidney J. 2013;6:8-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: A systematic review. BMC Nephrol. 2014;15:184.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Renal recovery after acute kidney injury. In: Ding X, Ronco C, eds. Acute kidney injury - from diagnosis to care. Chapter 24-35. 2016 Feb 08. [Last accessed on 2023 Jun 25]
- [Google Scholar]
- International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet Commissions. 2015;385:2626-43.
- [CrossRef] [PubMed] [Google Scholar]
- Cholera gravis associated with acute renal failure in a traveler from Haiti to the United States. Travel Med Infect Dis. 2012;10:236-9.
- [CrossRef] [PubMed] [Google Scholar]
- Update on hemolytic uremic syndrome: Diagnostic and therapeutic recommendations. World J Nephrol. 2013;2:56-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Crescentic infection related glomerulonephritis in adult and its outcome. Saudi J Kidney Dis Transpl. 2018;29:623-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hemolytic uremic syndrome in children in northern India. Pediatr Nephrol Berl Ger. 1991;5:284-8.
- [CrossRef] [PubMed] [Google Scholar]
- SIRS, qSOFA and new sepsis definition. J Thorac Dis. 2017;9:943-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diarrhoeal disease. Available from https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease [Last accessed on 2023 Jun 25].
- The requirement of hemodialysis in patients with acute gastroenteritis-induced acute kidney injury. J Fam Med Prim Care. 2021;10:2423-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute renal failure due to acute diarrhoeal diseases. J Assoc Physicians India. 1990;38:164-6.
- [PubMed] [Google Scholar]
- The treatment of cholera: Clinical science at the bedside. J Infect Dis. 1992;166:2-14.
- [CrossRef] [PubMed] [Google Scholar]
- Acute renal failure in eastern India. Nephrol Dial Transplant. 1995;10:2009-12.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolic acidosis in acute renal failure following acute diarrhoeal disease–An important prognostic factor? J Assoc Physicians India. 1992;40:553.
- [PubMed] [Google Scholar]
- Epidemiologic trend changes in acute renal failure--a tertiary center experience from South India. Ren Fail. 2006;28:405-10.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury in a tertiary care center of South India. Indian J Nephrol. 2022;32:206-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







