Translate this page into:
Posterior reversible encephalopathy syndrome in children with kidney disease
Address for correspondence: Dr. Dinesh Gera, Department of Nephrology and Clinical Transplantation, Institute of Kidney Diseases and Research Centre - Dr HL Trivedi Institute of Transplantation Sciences [IKDRC-ITS], Civil Hospital Campus, Asarwa, Ahmedabad - 380 016, Gujarat, India. E-mail: dinesh.gera@ikdrc its.net
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Posterior reversible encephalopathy syndrome (PRES) is a clinic-radiographic entity of heterogeneous etiologies that are grouped together because of similar findings on neuro-imaging and associated symptom complex of headache, vision loss, altered mentation, and seizures. Although usually considered benign and reversible, characteristics of this syndrome in pediatric patients remain obscure. This case series included 11 patients (8 males, 3 females, age 3-15 years) of PRES during September 2010 to February 2012 out of a total 660 renal pediatric patients (1.66%). We studied their clinical profile, contributory factors, and outcome. Presenting symptoms were headache in 73%, dimness of vision or cortical blindness in 36%, seizures in 91%, and altered mentation in 55%. The associated renal diseases were acute renal failure (55%), chronic renal failure (9%), and 36% had normal renal function. The contributory factors were uncontrolled hypertension (100%), severe hypoproteinemia (9%), persistent hypocalcemia (9%), hemolytic uremic syndrome (36%), cyclosporine toxicity (9%), lupus nephritis (9%), high hematocrit (9%), and pulse methylprednisolone (9%). Brain imaging showed involvement of occipito-parietal area (100%) and other brain areas (63%). All but one patient of hemolytic uremic syndrome had complete clinical neurological recovery in a week, and all had normal neurological imaging after 4-5 weeks. PRES is an underdiagnosed entity in pediatric renal disease patients. Associated hypertension, renal disease, and immunosuppressive treatment are important triggers. Early diagnosis and treatment of comorbid conditions is of prime importance for early reversal of syndrome.
Keywords
Contributory factors
course and time frame of recovery
pediatric renal patients
posterior reversible encephalopathy syndrome
radio-imaging
Introduction
Posterior reversible encephalopathy syndrome (PRES) was first reported as reversible posterior leucoencephalopathy syndrome in 1996.[1] Initially, it was described in acutely ill patients who had a reversible syndrome of headache, altered mental function, seizure, and loss of vision associated with findings indicating predominantly posterior leucoencephalopathy on imaging studies.[1] Literature consists of few case reports or few small series of patients in different clinical settings.[1234567] All age groups are susceptible to the syndrome. The setting in which it was described included patients with hypertensive encephathalopathies, patients who were receiving immunosuppressive medicines after organ transplantation or other malignancies, patients with renal failure, and patients with eclampsia. Other comorbid conditions later described included children with nephrotic syndrome, acute nephritis, hemolytic uremic syndrome, systemic lupus erythematosus, and patients with volume and electrolyte disturbances. Pathogenetic mechanism is believed to be vasogenic edema and hyperperfusion of cerebral white matter affecting commonly occipito-parietal region due to autoregulatory failure or endothelial dysfunction.[18] Predilection for posterior brain regions could be due to pial and intracerebral vessels in anterior circulation which have higher concentration of adrenergic nerves when compared with posterior hemisphere.[910] Diagnosis was made in appropriate clinical setting with neurological symptoms supported by neurological imaging by computed tomography (CT) scan brain, magnetic resonance imaging (MRI), and diffusion weighted images. We describe a series of children with kidney disease who developed PRES and describe their clinical and laboratory features.
Materials and Methods
Out of total 660 pediatric renal patients including renal transplant patients admitted at our institute during September 2010 to February 2012 (18 months), we identified 11 patients with characteristic neurological symptom complex in association with typical neurological images suggesting PRES as described in Introduction. All patients had undergone thorough evaluation clinically, neurologically, and biochemically to search for likely contributory factors with special attention to blood pressure, volume status, severity of anemia, presence or absence of hemolysis, and its severity by following peripheral smear, platelet count, LDH, level of renal function, electrolyte and acid base balance, severity of hypoproteinemia, character of underlying renal disease, and blood level of immunosuppressive medications. Hypertension was staged according to fourth Task Force report on high blood pressure in children and adolescents[11] by comparing patients BP reading to BP level for boys and girls by age and height percentile in BP table prepared by National Heart Lung and Blood Institute (NHLBI).[11] Grade 1 hypertension is defined as systolic or diastolic BP from 95 percentile to 99 percentile, +5 mm Hg for age and height for individual gender. Grade 2 hypertension is designated to BP levels that are more than 5 mmHg above 99 percentile for age and height for individual gender. Severity of acute renal failure was staged according to RIFLE criteria.[12]
Possibility of other neurological disorders like cerebral venous thrombosis, meningeal infection, intracranial hemorrhage or space occupying lesion were ruled out in each case with help of appropriate investigations. Initially, all patients were evaluated by in-house cranial CT scan without contrast. MRI with or without contrast was obtained in patients where CT brain alone was inconclusive. MR venography was performed where the possibility of cerebral venous thrombosis was considered. Imaging was studied in collaboration with hospital radiologists involved in the study. Lumbar puncture and CSF study was performed in selected cases.
All patients received appropriate treatment for renal disease, adequate dialysis for renal failure, timely correction of fluid electrolyte and acid base balance, Nitroglycerine drip and other anti-hypertensives for hypertension, IV albumin for hypoalbuminemia, plasma exchanges in cases of HUS, and dose adjustment of the medication was made. Record of Treatment received for renal disease and prevailing abnormal parameters and time frame of clinical and biochemical improvement was made and follow-up radio-images were compared (usually taken at 4 week interval and more frequently in selected cases) with original images.
Results
A total of 11 patients (eight boys and three girls, ranging in age from 3 years to 15 years) with PRES, were evaluated and studied. Clinical and biochemical characteristics and cranial imaging findings is shown in Table 1. Presenting symptoms were seizures in ten cases (91%), headache in eight cases (72%), altered mental status in five cases (45%), and dimness of vision or cortical blindness in three cases (27%) in different combinations. So far as underlying renal disease and setting of syndrome complex is considered among these patients, six patients (55%) had acute renal failure (four had HUS, one had pauci-immune crescentic GN, one had solitary functioning kidney obstructed by a stone who developed PRES in setting of post-obstructive diuresis and associated persistent hypocalcemia), four patients (36%) had normal renal function [one patient with diffuse proliferative Lupus nephritis and uncontrolled hypertension, one patient of focal segmental glomerulosclerosis (FSGS) with high trough level of cyclosporine 300 ng/ml, one patient with MPGN who developed hypertensive encephalopathy due to non-compliance to medication, one with morbid nephrotic state with scrotal skin necrosis and severe hypoproteinemia (serum albumin 1.1 gm/dL)]. One patient of chronic renal failure (CRF) who was on continuous ambulatory peritoneal dialysis (CAPD) developed PRES after receiving inadvertent blood transfusion due to misjudged anemia and his Hb at the time of PRES was 12.5 gm/dL.

Significant comorbid contributory factors found in these patients at the time of PRES were uncontrolled hypertension in all 11 patients (100%), renal failure (55%), CNI toxicity (9%), hypoproteinemia (9%), high hematocrit (9%), hypocalcemia (9%), steroid toxicity (9%), HUS (36%), and lupus nephritis (9%) [Figure 1]. A few illustrative cases are described below.

- Case no. 5 showing morbid nephrotic state with scrotal skin necrosis
Case 1
A five-year-old-male child weighing 14 kg was admitted with ARF and serum creatinine of 6.4 mg/dl, and respiratory acidosis. He was prepared by acute peritoneal dialysis for PCN diversion of obstructed solitary functioning kidney. Postoperatively after 12 h of diversion child developed convulsion. Clinically, his pulse and hydration was normal and BP was 130/90 mmHg (grade 2 HT) which was controlled with oral nifedipine. Biochemically, electrolytes were normal except for ionized serum calcium which was 2.26 mg/dl, phosphate 5.4 mg/dl, Magnesium 1.77 mg/dl, pH of 7.32 and serum bicarbonate level was 17.5 meq/L. Patient was treated by sodium bicarbonate drip in one IV line and calcium gluconate drip in another IV line. In first 12 h the child received 15 ml of calcium gluconate and 10ml in next 12 h. He was given 0.5 ml of magnesium sulphate (i.e., 250 mg; 25% w/v) intramuscularly. The child's venous blood gas and electrolytes were studied every 6 hours and child persisted to be severely hypocalcemic though he received 15 ml of calcium gluconate on second day and 10 ml for further two days. So in total 50 ml of calcium gluconate was given in three days of PCN diversion in a 14 kg child. Childs hypocalcemia was within normal range on fourth day only when we reduced 24 h calcium dose in stepwise fashion. His BP was well controlled with oral nifedipine. Throughout the course, his urine output, serum urea, and serum creatinine were improving day by day after diversion. We measured 24 h urinary calcium also on 2nd day after diversion which was 30 mg/24 h which was high in face of very low ionized serum calcium level. The amount of calcium gluconate the child required parenterally to correct hypocalcemia was surprisingly high which we have observed first time in such a setting. His iPTH, five days after PCN diversion was 408 pg/ml which was 540 pg/ml when he presented with ARF. Logically, we hypothesized that calcium gluconate given iv was increasingly being taken up by bone due to decreasing hyperparathyroidism in association with improving renal function and it was wasted to some extent in urine also because of defect in tubular reabsorption leading to persistent hypocalcemia. Calcium has unique intracellular messanger role and it is complexed with numerous ligands in cytosol, including adenosine and vitamin D dependent calcium binding protein, calmodulin.[13] Persistent hypocalcemia may be expected to affect directly or indirectly mechanisms involved in autoregulation and endothelial function.
Case 2
An 8-year-old child, weighing 15 kg (CRF on CAPD) was found to have PRES with history of blood transfusion by private pediatrician. His Hb was 12.5 g% with grade 2 HT (BP 120/80 mm Hg) when he presented to us with PRES. Initially, we also intensified PD with 2.5% dextrose CAPD fluid, used diuretics, and controlled HT with oral medication for two days. His urine output was also more than 400 ml. Child was on CAPD for 1.5 year without any neurological complaint. During his intensive PD with hypertonic fluid, child lost 300 g weight after 1st day and further 150 g on 2nd day after admission. Child was euvolemic with normal X-ray chest and was not improving neurologically for two days. So, observing the temporal relationship of blood transfusion and development of PRES, we tapped 100 ml of blood on 3rd day by femoral route and PD was switched to 1.5% Dextrose solution again. Temporal relationship of improvement with phlebotomy left no doubt about the fact that blood transfusion was causally related to PRES in this child who was habituated to low hematocrit. Sudden rise in hematocrit might have resulted in increased viscosity of blood and rise in vascular resistance, superimposed on possibly sensitive cerebral vasculature, may have resulted in hypertensive encephalopathy like picture. There are case reports in literature also about PRES after blood transfusion.[14] KDOQI clinical practice recommendation for anemia in CKD also have set target of Hb in the range of 11-12 g/dl.[15]
Initial cranial imaging revealed fairly symmetrical areas of edema not only involving occipito-parietal white matter regions in majority of cases but also extended to cortical gray matter in nine cases (81%) and extended to fronto-temporal area in five cases (45%), involved basal ganglia also in two patients (case 4 and 11) whereas cerebellum and medulla were also involved in one patient (case 11) [Figure 2].
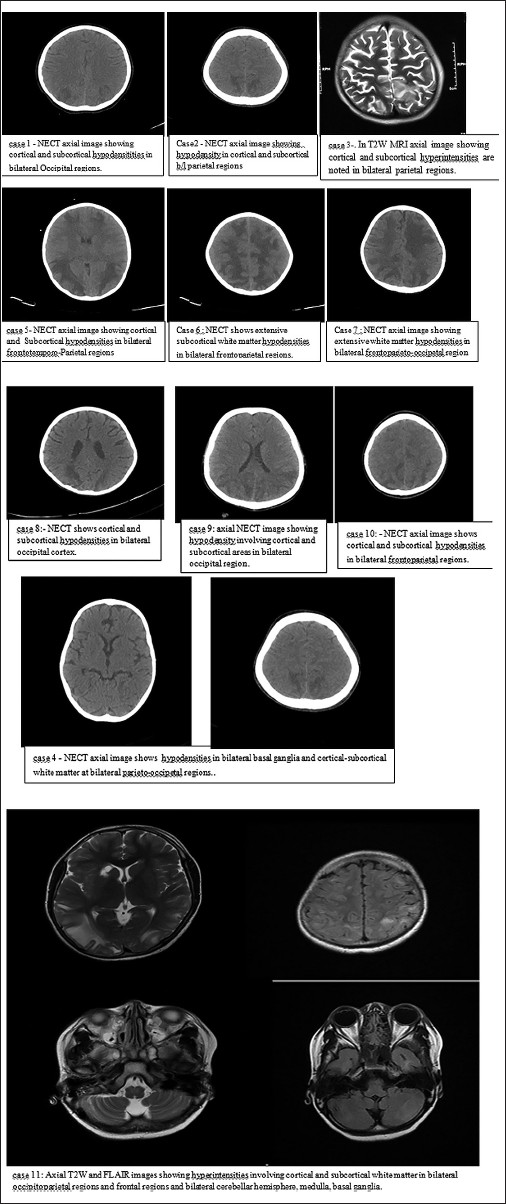
- Computed tomography/Magnetic resonance imaging images of brain showing positive findings
All patients had neurological recovery within 5-7 days except one patient of HUS (case four) who persisted to have altered mentation even after a month of follow up, though his renal function and cranial imaging had recovered completely after a month. This was the patient who had neurological symptoms for six days before getting admitted in our hospital where specific management of peritoneal dialysis and plasma exchange was started.
Follow-up cranial images were normal after four to five weeks in all cases. All patients had normal renal function after four weeks except in two cases, one with HUS who was put on CAPD and one patient with obstructed solitary functioning kidney who had serum creatinine of 1.9 mg/dl at the end of one month when he was stone free.
Discussion
With easy availability of radio-imaging technique, MRI in particular, there is increasing number of reports of PRES in all age groups including pediatric patients over last ten years.[1617] With an objective of studying predisposing factors, clinical spectrum, cranial imaging, and course of PRES in pediatric renal patients, present study was conducted. Renal patients are at particular risk of PRES because renal disease and hypertension go hand in hand. Higher chances of volume perturbation, electrolyte disturbances and mechanism of renal disease itself, its treatment-associated drug toxicities, and other complications of management put these patients at special risk for PRES.
Table 2 shows comparison of our results with two other pediatric studies of PRES in literature. It could be seen that in all three pediatric series there were more boys than girls with PRES. Other two pediatric series, recently published, also showed more boys than girls.[1617] In adult series, females were affected more frequently than males even when eclampsia cases were excluded.[1618]

Seizure as a presenting symptom was most common in Ishikura's study (85%) as well as in our study (91%). All the series had different combination of four clinical characteristic symptoms as a presenting picture. So far as level of renal function was considered among these patients, we found six patients (55%) with acute renal failure. Other two studies have not specified about level of renal function. But more and more patients with hypertension and renal failure of different etiologies are reported with PRES.[16]
All three studies have associated hypertension in more than 95% of their cases. Hypertension was almost always observed in PRES but its level was not correlated to the severity of PRES and cause effect relationship is yet to be established, as in many cases other comorbid conditions like drug toxicity, vacuities, auto-immune disease, or endothelial dysfunction from any cause may be involved.[192021222728]
Nephrotic state is a special risk for PRES and may have multiple factors involved because they are often hypertensive, receive steroid and CNI, may have severe hypoproteinemia, and may have massive oedema with fluid retention and associated increased vascular permeability.[2930] Ishikura's series had seven cases (35%), Kwon series had two cases (16%) whereas our study had two cases (18%) of nephrotic syndrome.
Like our series (36% HUS), series of Kwon et al. also had two cases of HUS (16.5%) who developed PRES. Here, pathological process itself in addition to renal failure and hypertension could be involved in pathogenesis like that of pre-eclampsia.[20]
Our patient with pauci-immune crescentic GN (case 1) had developed PRES following three pulses of methylprednisolone. High dose steroid was also found associated in study by Kwon et al., in two cases (16%) of nephrotic syndrome, whereas no specific allegation to steroid high dose was made in Ishikura series.
Ishikura's Series had ten patients (50%) with kidney transplantation on CNI and Kwon et al. 33% of cases on CNI and our study had 9% (1 case) of PRES which was associated with CNI overdose. Immunosuppresive medicines and CNI in particular are more frequently reported to be associated with PRES in post-transplant scenario. After renal toxicity, vascular toxicity, and neurotoxicity are the most serious side effect of CNI, affecting 25-59% of transplant patients.[23] Reduction in drug dosage or prompt withdrawal of cytotoxic drugs is usually recommended in cases of PRES.[2425] When same agent is re-introduced, patient must be followed closely as recurrence has been reported in this setting.[26]
Recently, de Laat from Netherlands described seven childhood cancer patients with clinical radiological findings of PRES.[16] He also reviewed 49 other documented cases of PRES from literature during childhood cancer and concluded that hypertension and renal failure related to immunosuppressive treatment of leukemia and other solid tumor seems to be the most important triggers for occurrence of PRES.[16] Similarly, Faruk Inceik from Turkey in 2009 described nine cases of pediatric PRES of whom seven were receiving immunosuppressive treatment and two were hypertensive crisis patients. Four patients received intrathecal injections of immunosuppressive drugs for different reasons and developed PRES.[17]
Involvement of occipital and parietal region was almost universal in all three studies and lesions were not restricted to white matter alone but involved gray matter also in majority of cases in all three studies. Other areas like frontal lobe, temporal lobe, basal ganglia, cerebellum, and medulla were involved in lesser frequency in all series with individual incidence.
Follow-up cranial images were normal after four to five weeks in all cases in present study. One case of HUS (9%) persisted to have altered mentation even after a month of follow-up, though his renal function and cranial imaging had recovered completely. In a study by Ishikura, also 90% had complete recovery and 10% had neurological sequel. Kwon et al. also had complete neurological recovery in 92% of cases.
Conclusion
PRES is not uncommon in pediatric nephrology. Hypertension, renal disease, immunosuppression, and chemotherapy of malignancies are triggers for PRES. It is important to consider this diagnosis in children presenting with seizure, visual disturbances, headache, and altered mentation in appropriate clinical setting.
Source of Support: Nil
Conflict of Interest: None declared.
References
- A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
- [Google Scholar]
- Posterior reversible encephalopathy syndrome in children: Its high prevalence and more extensive imaging findings. Am J Kidney Dis. 2006;48:231-8.
- [Google Scholar]
- Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol. 2001;24:361-4.
- [Google Scholar]
- Nephrotic state as a risk factor for developing posterior reversible encephalopathy syndrome in paediatric patients with nephrotic syndrome. Nephrol Dial Transplant. 2008;23:2531-6.
- [Google Scholar]
- Cortical blindness in a child with acute glomerulonephritis. Indian J Nephrol. 2012;22:42-4.
- [Google Scholar]
- Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205-10.
- [Google Scholar]
- Cyclosporin-A induced posterior reversible encephalopathy syndrome. Saudi J Kidney Dis Transpl. 2008;19:439-42.
- [Google Scholar]
- Diffusion-weighted MR imaging in hypertensive encephalopathy: Clues to pathogenesis. AJNR Am J Neuroradiol. 1998;19:859-62.
- [Google Scholar]
- Cerebral circulation in acute arterial hypertension–protective effects of sympathetic nervous activity. Acta Physiol Scand. 1981;111:193-9.
- [Google Scholar]
- Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res. 1976;115:377-93.
- [Google Scholar]
- The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. National high blood pressure education program working group on high blood pressure in children and adolescents. Pediatrics. 2004;114:555-76.
- [Google Scholar]
- Defining acute renal failure: The RIFLE criteria. J Intensive Care Med. 2007;22:187-93.
- [Google Scholar]
- Disorders of calcium and magnesium and phosphate balance. In: Barry M, Brenner MD, eds. The Kidney (4th edition). Philadelphia, PA: WB Saunders; p. :841-87.
- [Google Scholar]
- Seizures related to blood transfusion and erythropoietin treatment in patients undergoing dialysis. BMJ. 1989;299:1258-9.
- [Google Scholar]
- KDOQI. KDOQI Clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471-530.
- [Google Scholar]
- Posterior reversible encephalopathy syndrome in childhood cancer. Ann Oncol. 2011;22:472-8.
- [Google Scholar]
- Evaluation of nine children with reversible posterior encephalopathy syndrome. Neurol India. 2009;57:475-8.
- [Google Scholar]
- Reversible posterior leukoencephalopathy syndrome: A misnomer reviewed. Intern Med J. 2005;35:83-90.
- [Google Scholar]
- Posterior reversible encephalopathy syndrome, part 2: Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043-9.
- [Google Scholar]
- Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28:1320-7.
- [Google Scholar]
- Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27:2179-90.
- [Google Scholar]
- Posterior leukoencephalopathy without severe hypertension: Utility of diffusion-weighted MRI. Neurology. 1998;51:1369-76.
- [Google Scholar]
- Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. J Biol Chem. 2002;277:29669-73.
- [Google Scholar]
- Preeclampsia-eclampsia: Clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217:371-6.
- [Google Scholar]
- Delayed peripartum vasculopathy: Cerebral eclampsia revisited. Ann Neurol. 1993;33:222-5.
- [Google Scholar]
- Nephrotic state as a risk factor for developing posterior reversible encephalopathy syndrome in paediatric patients with nephrotic syndrome. Nephrol Dial Transplant. 2008;23:2531-6.
- [Google Scholar]
- Molecular mechanism of edema formation in nephrotic syndrome: Therapeutic implications. Pediatr Nephrol. 2007;22:1983-90.
- [Google Scholar]







