Translate this page into:
Prevalence of Clinically Significant anti-HLA Antibodies in Renal Transplant Patients: Single-center Report from North India
Address for correspondence: Ms. Rajni Chauhan, Amity Institute of Biotechnology, Amity University Uttar Pradesh, Sector 125, Noida - 201 301, India. Email: rajnichauhan22@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Solid organ transplantation is the preferred therapeutic modality of treatment in patients affected by terminal organ failures. Human leukocyte antigens (HLAs) plays an important role in graft survival. In many of the cases of rejection, antibodies are directed against HLA antigens expressed on the cells of the transplanted organ. Pre-transplant compatibility testing involves the use of different methodologies for the determination of anti-HLA antibodies. Luminex single-antigen bead (SAB) assay demonstrates higher sensitivity and specificity in detecting anti-HLA antibodies. The aim of this study was to determine the prevalence of anti-HLA antibodies in pre-transplant work up recipients, planned for renal transplant at a tertiary care center in India.
Methods:
1640 patients visiting tertiary care hospital for pre-transplant compatibility testing were screened with complement-dependent micro-lymphocytotoxicity crossmatch (CDC-XM) and flow cytometric crossmatch (FC-XM). The patients positive for either or both screening tests were assayed with the Luminex SAB tests in order to establish defined antigen specificity of the alloantibodies and determining donor-specific antibody (DSA).
Results:
The two most frequent antibodies identified in each A, B, C locus of HLA class I were -A*24:03 (43.9%), A*25:01 (36.6%), B*57:01 (40.3%), B*15:12 (37.1%), C*17:01 (61.9%), C*07:01 (52.4%) and in DR, DQ DP locus in HLA class II were DRB1*09:01(40.0%), DRB1*14:04(37.6%), DQA1*04:01/DQB1*03:03 (58.4%), DQA1*05:01/DQB1*03:01 (55.1%), DPA1*02:01/DPB1*17:01 (55.0%), DPA1*02:01/DPB1*05:01 (45.0%).
Conclusion:
This study has found the prevalence and specificity of anti-HLA antibodies in north India.
Keywords
CDC crossmatch
DSA
HLA
renal transplant
single-antigen bead assay
Introduction
Human leukocyte antigen (HLA) class I and class II can be genetically extremely diverse with a total of 23,907 alleles reported in the international immunogenetics information system (IMGT) database[1] in July 2019. HLA plays a central role in immunity and are of primary importance in organ transplantation. Anti-HLA antibody production occurs as a result of sensitizing events like blood transfusion(s), pregnancy or previous transplant(s).[2] Preformed antibodies against HLA may lead to antibody-mediated rejection (AMR) and remain a significant barrier in clinical renal transplantation.[3] Detection of HLA antibodies, particularly donor-specific antibodies (DSA) is a very crucial step in pre-transplant assessment[4] for optimal donor selection and graft survival. The cell-based assay like complement-dependent micro-lymphocytotoxicity cross-match (CDC-XM) was introduced in the 1960s for detecting HLA antibodies in transplant patients.[5] CDC-XM and antibody detection techniques have improved over time with higher sensitivity and specificity.[65678] Several modifications of CDC-XM [anti-human globulin CDC cross-match (AHG-CDC-XM) and dithiothreitol CDC cross-match (DTT-CDC-XM)] were introduced over the years to obtain higher sensitivity. Simultaneously, with the advent of time, other methodologies based on flow-cytometry (flow cytometric cross-match [FC-XM]) and Luminex (single antigen bead [SAB]) assays came into practice.[91011] Luminex SAB assay demonstrates higher sensitivity and specificity in detecting anti-HLA antibodies among all methodologies and hence used by many transplant centers for the detection of anti-HLA antibodies.[12] The aim of this study was to determine the prevalence of anti-HLA antibodies detected by SAB assay in renal transplant recipients at a tertiary care center in north India.
Material and Methods
Settings
This retrospective study was done from March 2015 to May 2018 at the molecular and transplant immunology laboratory in a tertiary health care center in the national capital region of India, which primarily caters to the north Indian patient population. Patient registration included residential addresses and patients from northern states (Rajasthan, Madhya Pradesh, Chhattisgarh, Jharkhand, Bihar, Uttar Pradesh, Uttaranchal, Himachal Pradesh, Punjab, and Haryana) northern union territories (Ladakh, Jammu, Srinagar, Chandigarh, and Delhi) were included. A total of 1640 patients were evaluated for pre-transplant compatibility workup. In accordance with the THOTA,[13] 2014, all patient-donor pairs underwent human leukocyte antigen (HLA) typing to prove the relationship. AHG-CDC-XM and FC-XM were the screening tests and any recipient positive for either of screening cross-match tests underwent SAB assay (n = 200). All 200 samples were analyzed for both classes I and class II anti-HLA antibodies. The clinical history including the number of blood transfusions, pregnancy, and the previous transplant was recorded for all the patients. Out of these 200 patients, 164 patients had a history of sensitization.
Technique and equipment
SAB assay was performed using Lifecodes® LSA Class I and Lifecodes® LSA Class II kits (Immucor, Inc., GA, US) on Luminex® 200 platform (Luminex Corporation, Austin, Texas, United States) using ×MAP technology. Beads included class I (HLA-A, HLA-B, HLA-C) and class II (HLA-DR, HLA-DQ) antigens. Antibody specificity and strength were analyzed with MATCH IT antibody software (Immucor GTI Diagnostics, Inc, Waukesha, WI, US). Individual bead raw median fluorescence intensity (MFI) value (>1000 MFI), background-corrected MFI (BCM), background-corrected ratio (BCR), and antigen density-BCR (AD-BCR) were calculated. As per the manufacturer's protocol, the bead was considered positive if two or more of these adjusted values were above the cutoff values. The cutoff value for a positive DSA was an MFI of ≥1000.
Statistical analysis
To summarize the data, counts, and percentages were used for categorical variables. Percentages and frequencies were calculated using Microsoft Office Standard 2013. The P-value <0.05 was considered statistically significant.
Ethical approval
Patient consent was obtained for diagnosis and treatment at the hospital. This was an observational study; no personal identifiers like name, addresses were used, and no additional sample was drawn for this study. All investigation, treatment, and monitoring were according to the current “standard-of-care.”
Results
A total of 1640 patients who came for pre-transplant workup between March 2015 and May 2018 were included in the study. Among those, 200 (19.2%) patients (147 males and 53 females) underwent SAB testing. Off 200 serum samples tested for HLA antibodies, 113 (56.5%) were positive for HLA-class I antibodies and 135 (67.5%) were positive for HLA-class II antibodies.
Out of 87 patients which were negative for HLA class I antibody, 69 (79.3%) were males, out of which 51 (73.9%) had a history of sensitization and 18 (20.7%) were females, out of which 17 (94.4%) %) had a history of sensitization. Among 113 patients who were positive for HLA class I antibody, 78 (69.1%) were males, out of those 64 (82.1%) had a history of sensitization and 35 (30.9%) were females, out of those 32 (91.4%) had a history of sensitization [Table 1].
| HLA class I (200) | HLA class II (200) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | P* | Gender | P* | |||||||
| Negative (87) | Positive (113) | Negative (65) | Positive (135) | |||||||
| Sensitization status | Male 69 (79.3) | Female 18 (20.7) | Male 78 (69.1) | Female 35 (30.9) | Male 56 (86.2) | Female 9 (13.8) | Male 91 (67.4) | Female 44 (32.6) | ||
| Transfusion | 22 (31.8) | 7 (38.8) | 38 (48.7) | 21 (60.0) | 0.00758 | 18 (32.1) | 4 (44.4) | 42 (46.2) | 24 (54.5) | 0.04444 |
| Pregnancy | NA | 10 (55.5) | NA | 21 (60.0) | NA | NA | 3 (33.3) | NA | 28 (63.6) | NA |
| Previous transplant | 40 (57.9) | 10 (55.5) | 35 (44.9) | 11 (31.4) | 0.01878 | 25 (44.6) | 5 (55.5) | 50 (54.9) | 16 (36.4) | 0.71884 |
| Overall sensitization | 51 (73.9) | 17 (94.4) | 64 (82.1) | 32 (91.4) | 0.21498 | 40 (71.4) | 8 (88.8) | 75 (82.4) | 41 (93.2) | 0.03752 |
Out of 65 patients which were negative for the HLA class II antibody, 56 (86.2%) were males, out of which 40 (71.4%) had a history of sensitization and 9 (13.8%) were females, out of which 8 (88.8%) had a history of sensitization. Among 135 patients which were positive for HLA class II antibody, 91 (67.4%) were males, out of those 75 (82.4%) had a history of sensitization and 44 (32.6%) were females, out of those 41 (93.2%) had a history of sensitization [Table 1].
Among 113 HLA class I antibodies positive patients, 49 patients had antibodies against single locus [32 (28.3%) against HLA-A, 12 (10.6%) against HLA-B, 5 (4.4%) against HLA-C] and 53 had antibodies against multiple-locus. Thirty-seven (32.7%) had against HLA-A and HLA-B, 3 (2.7%) against HLA-B and HLA-C, 3 (2.7%) against HLA-A and C, and 10 (8.8%) against all the three classes (HLA-A, B, and C) [Figure 1a].

- (a) Frequencies of Human leukocyte antigen (HLA) class I antibodies against single and multiple loci. (n = 200); (b). Frequencies of HLA class II antibodies against single and multiple loci (n = 200)
Out of 200 cases tested for HLA class II antibodies, 135 (67.5%) were positive and 65 (32.5%) were negative. 21 (15.6%) were positive for HLA-DR, 26 (19.3%) for HLA-DQ and 8 (5.9%) for HLA-DP. Thirty-seven (27.4%) had antibodies against both DR and DQ, 6 (4.4%) against HLA-DR and HLA-DP, 5 (3.7%) against HLA-DP and HLA-DQ and 21(15.6%) against all the three class II antigens (HLA-DR, HLA-DQ and HLA-DP) [Figure 1b].
The five most common antibodies identified in HLA class I were A*24:03 (43.9%), A*25:01 (36.6%), A*02:02 (35.4%), A*24:02 (35.4%), A*02:05 (34.1%) in A locus, B*57:01 (40.3%), B*15:12 (37.1%), B*44:03 (35.5%), B*44:02 (33.9%), B*58:01 (33.9%) in B locus, C*17:01 (61.9%), C*07:01 (52.4%), C*07:02 (47.6%), C*18:01 (38.1%), C*04:03 (33.3) in C locus. In HLA class II, the five most common antibodies were DRB1*09:01 (40.0%), DRB1*14:04 (37.6%), DRB1*04:01 (31.8%), DRB1*11:01 (31.8%), DRB1*11:04 in DR locus, DQA1*04:01/DQB1*03:03 (58.4%), DQA1*05:01/DQB1*03:01 (55.1%), DQA1*06:01/DQB1*03:01 (50.6%), DQA1*04:01/DQB1*04:02 (49.4%), DQA1*05:01/DQB1*04:01 (49.4%) in DQ locus, DPA1*02:01/DPB1*17:01 (55.0%), DPA1*02:01/DPB1*05:01 (45.0%), DPA1*01:03/DPB1*18:01 (45.0%), DPA1*02:01/DPB1*14:01 (40.0%), DPA1*01:03/DPB1*02:01 (35.0%) in DP locus. The ten most common anti-HLA antibodies identified against HLA Class I (A, B, C) and HLA Class II (DRB1, DQB1, DPB1) antigens are shown in Figure 2a and b.
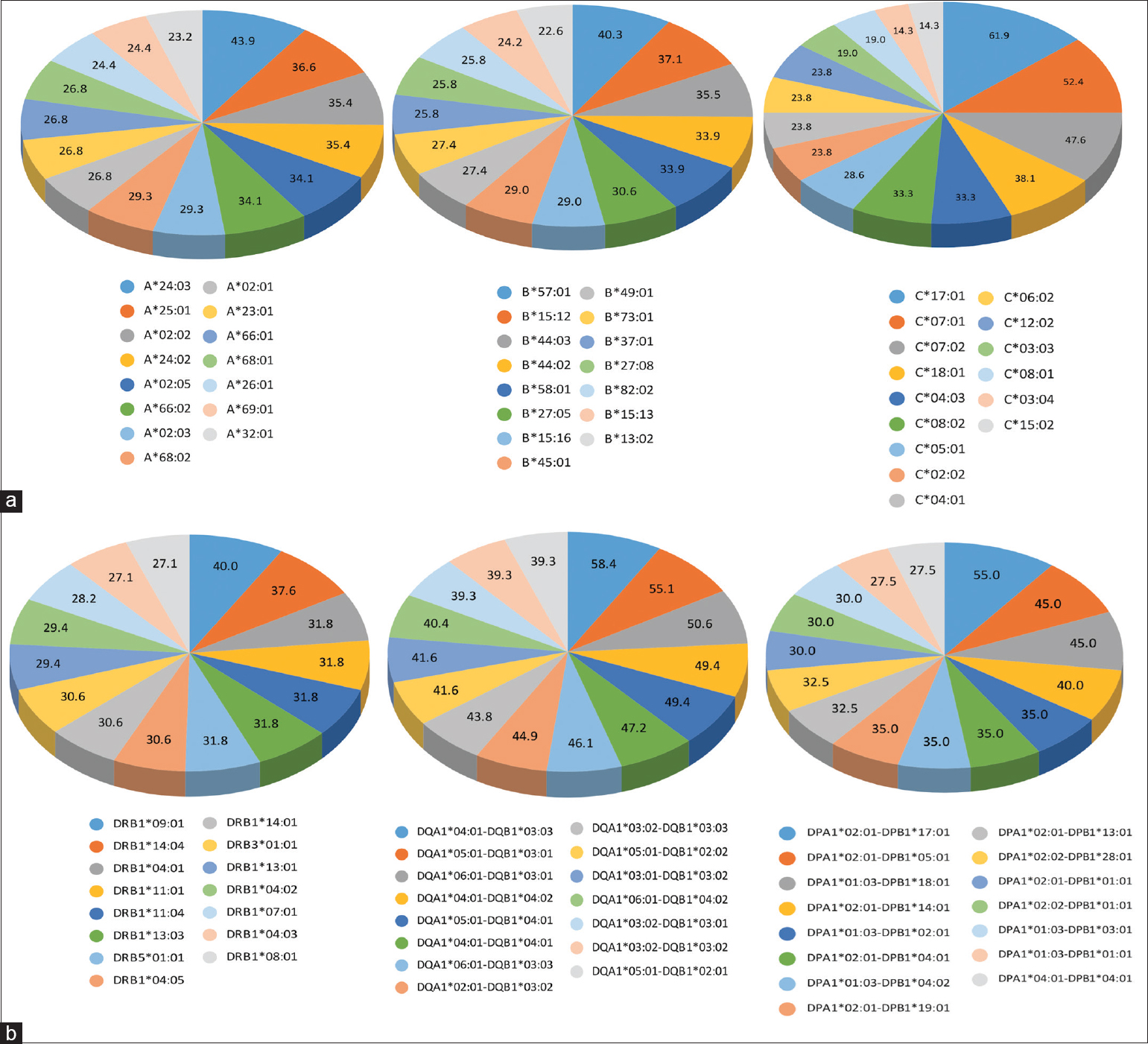
- (a) Distribution of the 15 most common anti-HLA antibodies identified against class I antigens (A, B, and C). (b) Distribution of 15 most common anti-HLA antibodies identified against class II antigens (DR, DQ, and DP)
Discussion
The primary aim of this study was to estimate, the prevalence of anti-HLA antibodies in patients requiring renal transplants. Renal transplantation rates in resource-constrained countries like India are considerably lower than in the developed world. One of the important reasons is absence transplant units with adequately trained staff, availability of donor and cost of therapy.[14] Anti-HLA antibody prevalence statistics would be useful for establishing a new “transplant immunology” laboratory and 'clinical transplant unit'. The paucity of data on prevalence and type of anti-HLA antibodies encouraged authors to perform, a study to fill this gap. DSA is a major risk factor for early renal allograft rejection and graft loss.[1516] Pre-transplant compatibility testing involves a combination of tests like CDC-XM, FC-XM, and SAB assay for the detection and identification of anti-HLA antibodies. In recent years, various methods have been developed to determine these antibodies in order to improve graft survival. The introduction of single-antigen bead (SAB) assays for detection and quantization of HLA antibodies has improved our ability to identify and manage allo-sensitized transplant candidates and recipients and to improve organ allocation.[17]
Our study may possibly be one of the first such studies conducted in India. There is a paucity of literature in this arena. In this study, sensitization history is directly proportional to the presence of anti-HLA antibodies in most of the sensitization categories (P < 0.05), as depicted in Table 1. As shown in Table 2, many alleles of our study are similar to study by Mishra et al., which may be due to the same population (Indian)[18] from which the subjects are drawn. At the antigenic level, when we compared our study with the Turkish population, similar antigens were observed in A, C, DR, and DQ locus.[19] Some antibodies present in the current study were similar in A, B, C, DQ, and DP locus at the allelic level with study in the Korean population.[20] The differences observed in another locus may be due to the diversity of HLA in the Asian subcontinent in which HLA A*02, B*40 and DRB1*15 are the common alleles, mainly reported from India and Pakistan.[21]
| Present study | Mishra et al., 2019 (Indian population) | Baştürk et al., 2016 (Turkish population*) | Park et al., 2018 (Korean population) |
|---|---|---|---|
| HLA Class I antibodies | |||
| A*24, A*25, A*02 | A*24, A*02 | A02, A68, A24 | A*02, A*11, A*33, A*66, A*80 |
| B*57, B*15, B*44, B*58 | B*15, B*27, B*45, B*82, B*13 | B49, B7, B37 | B*08, B*14, B*15, B*45, B*82 |
| C*17, C*07, C*18, C*04 | C*07, C*06, C*17, C*03 | Cw4, Cw6, Cw1, Cw2, Cw7, Cw16 | C*02, C*12, C*16, C*17, C*18 |
| HLA Class II antibodies | |||
| DRB1*09, DRB1*14, DRB1*04, DRB1*11 | DRB1*09, DRB1*04, DRB1*13, DRB3*01 | DR7, DR14, DR11 | DRB1*01, DRB1*03, DRB1*04, DRB1*11, DRB1*14 |
| DQA1*04, DQA1*05, DQA1*06, DQB1*04, DQB1*03 | DQA1*01, DQA1*05, DQA1*04, DQA1*06, DQB1*6, DQB1*03 DQB1*04 | DQ8 (DQA1*02, DQA1*03, DQB1*03), DQ9 (DQA1*03, DQA1*02, DQA1*05, DQB1*02, DQ2 (DQA1*04, DQA1*03, DQA1*06, DQB1*03,) | DQA*05, DQA1*02, DQA1*06, DQA1*03 DQB1*02, DQB1*04, DQB1*03 |
| DPA1*01, DPA1*02, DPB1*02, DPB1*05, DPB1*14 DPB1*17, DPB1*18, | DPA1*01, DPA1*02, DPB1*02, DPB1*03, DPB1*14, DPB1*18, DPB1*28 | NA | DPA1*01, DPA1*02, DPA1*03, DPB1*01, DPB1*05, DPB1*06, DPB1*13 |
*HLA antibodies are shown at antigenic level
The limitation of the study is that it is from a single center. Other multi-centric studies with a bigger sample size are required to validate the findings of this study and to establish prevalence and antibody specificity of anti-HLA antibodies in India.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) have given their consent for their clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the team members of the molecular and transplant immunology laboratory of Medanta-The Medicity for their support.
References
- Available from: https://www.ebi.ac.uk/ipd/imgt/hla/stats.html
- Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735-9.
- [Google Scholar]
- Detection of HLA antibodies in organ transplant recipients–triumphs and challenges of the solid phase bead assay. Front Immunol. 2016;7:570.
- [Google Scholar]
- Solid phase assays for HLA antibody detection in clinical transplantation. Curr Opin Immunol. 2009;21:573-7.
- [Google Scholar]
- Immunological assessment of the transplant patient. In: In: Kidney Transplantation. New York, NY: Springer’; 2014. p. :23-4.
- [Google Scholar]
- Flow cytometry crossmatching as a predictor of acute rejection in sensitized recipients of cadaveric renal transplants. Clin transplant. 2000;14:167-73.
- [Google Scholar]
- Biomarkers in transplantation: Prospective, blinded measurement of predictive value for the flow cytometry crossmatch after negative antiglobulin crossmatch in kidney transplantation. Kidney Int. 2006;70:1474-81.
- [Google Scholar]
- A virtual crossmatch protocol significantly increases access of highly sensitized patients to deceased donor kidney transplantation. Transplantation. 2008;86:1864-8.
- [Google Scholar]
- Prediction of crossmatch outcome of highly sensitized patients by single and/or multiple antigen bead luminex assay. Transplantation. 2006;82:1524-8.
- [Google Scholar]
- The virtual crossmatch–a screening tool for sensitized pediatric heart transplant recipients. Pediatr Transplant. 2006;10:38-41.
- [Google Scholar]
- Clinical significance of anti-HLA antibodies detected by Luminex®: Enhancing the interpretation of CDC-BXM and important post-transplantation monitoring tools. Hum Immunol. 2009;70:595-9.
- [Google Scholar]
- Transplantation of human organs and tissues Act-”Simplified”. Indian J Transplant. 2018;12:84.
- [Google Scholar]
- Kidney transplantation in India: Challenges and future recommendation. MAMC J Med Sci. 2016;2:12.
- [Google Scholar]
- Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: Contraindication vs.risk. Am J Transplant. 2003;3:1488-500.
- [Google Scholar]
- Clinical relevance of circulating donor-specific HLA antibodies. Curr Opin Organ Transplant. 2010;15:462-6.
- [Google Scholar]
- Prevalence and specificity of anti-HLA antibodies in Indian patients–single–centre data! ISBT Science Series 2019
- [Google Scholar]
- The most common HLA alleles and anti-HLA antibodies to know for virtual cross-match. Exp Clin Transplant. 2016;14(Suppl 3):53-5.
- [Google Scholar]
- A method for determining based on the frequencies of HLA alleles of HLA antibodies that are often observed with single antigen bead assay. Transplantation. 2018;102:S626.
- [Google Scholar]
- Complexities and similarities of HLA antigen distribution in Asian subcontinent. Indian J Hum Genet. 2010;16:108-10.
- [Google Scholar]







