Translate this page into:
Profile of incident chronic kidney disease related-mineral bone disorders in chronic kidney disease Stage 4 and 5: A hospital based cross-sectional survey
Address for correspondence: Dr. Anna T. Valson, Department of Nephrology, Christian Medical College Hospital, Vellore - 632 004, Tamil Nadu, India. E-mail: annavalson@cmcvellore.ac.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Chronic kidney disease related-mineral bone disorder (CKD-MBD) has been poorly studied in pre-dialysis Indian CKD patients. We aimed to study the clinical, biochemical and extra skeletal manifestations of untreated CKD-MBD in pre-dialysis Stage 4 and 5 CKD patients attending nephrology out-patient clinic at a tertiary care hospital in South India. A hospital based cross-sectional survey including, demographic profile, history of CKD-MBD symptoms, measurement of serum calcium, phosphate, parathyroid hormone, 25 hydroxy vitamin D (25(OH) D) and alkaline phosphatase; lateral abdominal X-rays for abdominal aortic calcification (AAC) and echocardiography for valvular calcification (VC) was carried out. Of the 710 patients surveyed, 45% had no CKD-MBD related symptom. Prevalence of hypocalcemia, hyperphosphatemia, hyperparathyroidism (>150 pg/mL) and 25(OH) D levels <30 ng/mL was 66.3%, 59%, 89.3% and 74.7% respectively. Echocardiography was carried out in 471 patients; 96% of whom had VC (calcification score ≥1). Patients with VC were older and had lower 25(OH) D levels than those without. Lateral abdominal X-rays were obtained in 558 patients, 6.8% of whom were found to have AAC, which was associated with older age. Indian patients with incident CKD-MBD have a high prevalence of hypocalcemia, 25(OH) D deficiency and VC even prior to initiating dialysis while AAC does not appear to be common. The association between 25(OH) D deficiency and VC needs further exploration.
Keywords
Aortic calcification
chronic kidney disease related mineral bone disorder
pre-dialysis chronic kidney disease
valvular calcification
vitamin D
Introduction
Chronic kidney disease (CKD) is now a public health problem affecting an estimated 10-13% of the world population.[12] As renal function declines, there is a progressive impairment in the regulation of mineral homeostasis leading to altered serum concentrations of calcium, phosphate, parathyroid hormone (PTH) and vitamin D. The end result of these biochemical abnormalities is disordered bone growth and remodeling and extraskeletal calcification; collectively known as chronic kidney disease related-mineral bone disorders (CKD-MBD).[3]
CKD-MBD is receiving widespread attention due its association with cardiovascular mortality.[4] Early detection and treatment of CKD-MBD is an important aspect of CKD management; however, CKD-MBD in the Indian population has been poorly studied. We therefore, sought to study the clinical and biochemical features as well as radiological manifestations of vascular calcification in untreated CKD-MBD among pre-dialysis CKD Stage 4 and 5 patients attending nephrology out-patient clinic at our tertiary health-care facility, catering to patients from a large part of the Indian subcontinent.
Subjects and Methods
Patients
This cross-sectional survey was conducted at the Department of Nephrology, Christian Medical College Hospital, Vellore, Tamil Nadu, India. Consecutive incident Stage 4 and 5 CKD patients attending nephrology out-patient clinic between November 2009 and August 2010 who satisfied both the following criteria were included:
-
Patients with newly diagnosed Stage 4 and 5 CKD (based on history, estimated glomerular filtration rate [eGFR] of <30 mL/min/1.73 m2 by the abbreviated modification of diet in renal disease formula, biochemical and ultrasonographic/histological evidence of CKD) who were not yet on dialysis or on hemodialysis/CAPD for <1 month at the time of enrolment in the study
-
Patients who had received calcium supplements/vitamin D analogs/phosphate binders for <3 months and had no prior history of calcimimetic use.
We excluded patients who had received steroids/cyclosporine/anticonvulsants for >3 months as well as patients on bisphosphonates. The study was approved by the Institutional Review Board and Ethics Committee of Christian Medical College, Vellore.
Figure 1 outlines the flow chart followed for patient selection. Each patient fulfilling criteria for inclusion in the study was interviewed to obtain information pertaining to demographic characteristics, socio-economic status (modified Kuppusamy Scale), CKD-MBD symptoms (bone pain, proximal muscle weakness, fragility fractures, pruritus and red eyes), approximate daily sunlight exposure, medication, dialysis and dietary history. Proximal muscle weakness was defined as difficulty in getting up from a squatting position in the absence of hypokalemia, hypophosphatemia and steroid use for >3 months. Fragility fractures were defined as fractures secondary to trivial trauma. Written informed consent was obtained in the appropriate format.

- Protocol followed for patient selection
Biochemistry
A fasting blood sample was drawn for measurement of biochemical parameters. PTH was measured in a simultaneously drawn plasma sample transported on ice to the laboratory, processed immediately and analyzed using a solid phase 2 site (1-34,44-84) chemiluminescent enzyme labeled immunometric assay (Immulite™, Diagnostics Products Corporation, CA, USA) with an intra and inter assay coefficient of variation of 5.7% and 8.8% respectively. 25 hydroxy vitamin D (25(OH) D) was measured in a fasting serum sample using the Elecsys modular analytics E170 immunoassay system (Roche Diagnostics, GmbH, Mannheim, Germany) with an intra- and inter-assay coefficient of variation of 5.7% and 7.1% respectively.
Normal values of serum calcium (corrected for albumin) and phosphate were defined as 8.5-9.5 mg/dL and 2.5-4.5 mg/dL respectively. Vitamin D deficiency, insufficiency and sufficiency were defined as <20 ng/mL, 20-30 ng/mL and >30 ng/mL. PTH level >150 pg/mL (2 times the upper limit of the assay) was labeled as hyperparathyroidism.
X-ray abdomen (lateral view)
Lateral abdominal X-ray to detect abdominal aortic calcification (AAC) was obtained according to a standard protocol and calcific lesions graded by a single radiologist blinded to clinical details using the index described by Kauppila et al.[5]
Echocardiography
Echocardiography was carried out specifically for the purpose of this study, with patient in the left lateral decubitus position, using the parasternal long and short axis views, by a single cardiologist blinded to all clinical details. Calcification was defined as the presence of echo brightness exceeding that of normal valve tissue. A global calcification score was obtained by semi-quantitative assessment of valvular calcification (VC) at the following 8 sites as described by Pressman et al.[6]
-
Posterior annulus, by thirds (score 0-3)
-
Posterior mitral leaflet restriction (0 = absent, 1 = present)
-
Anterior annulus calcification (0 = absent, 1 = present)
-
Anterior mitral leaflet restriction (0 = absent, 1 = present)
-
Mitral valve (MV) calcification (0 = absent, 1 = mild, 2 ≥mild)
-
Sub-valvular apparatus calcification (0 = absent, 1 = present)
-
Aortic valve (AV) calcification (0 = absent, 1 = nodule in <3 leaflets, 2 = nodules in 3 leaflets, non-restrictive; 3 = restrictive)
-
Aortic root calcification (0 = absent, 1 = present).
VC was defined as a calcification score ≥1 and significant VC was defined as a calcification score ≥5.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences version 16 (SPSS, Chicago, IL) as follows: Mean ± S.D were used for normally distributed data and median and interquartile range for skewed data. Significance was determined by Two Independent sample t test for continuous data, Chi-square test for categorical data and Mann-Whitney U Test for skewed data. PTH and 25(OH) D, which had skewed distributions, were log transformed and Pearson's coefficients (for continuous variables with normal distribution) and Spearman's rank correlation coefficients (for categorical and non-normally distributed variables) were used to define their correlation with various clinical and biochemical parameters. Univariate analysis was performed using simple linear regression at 25% level of significance and the variables obtained were incorporated in the adjusted analysis using stepwise multiple linear regressions to arrive at the final models. The β coefficients obtained were expressed as the percentage change in the dependent variable for a unit change in the independent variable using the transformation (eβ-1) × 100. To determine the clinical and demographic variables associated with VC and AAC, univariate analysis using binary logistic regression at 25% level of significance was carried out and variables which were significant were used to construct models using stepwise multivariate binary logistic regression. A P < 0.05 was considered to be statistically significant for the adjusted analysis.
Results
Demographic profile
The study population of 710 patients comprised adults predominantly belonging to the lower and middle socio-economic strata with a mean age of 46.6 ± 13.4 years, of whom 36.8% were diabetic, 81% were hypertensive and males outnumbered females 2.7:1. No exposure to dialysis was present in 92%. Diabetic nephropathy was the most common native kidney disease, followed by chronic glomerulonephritis; however in 58.2% the native kidney disease was unknown due to late referral. Only 31.7% and 20.6% had received calcium supplements/calcitriol and phosphate binders respectively, indicating a largely treatment naïve cohort. The median daily milk consumption and sunlight exposure, which are correlates for nutritional vitamin D, were 100 mL and 1 hour respectively. Patients with CKD Stage 5 were younger, less likely to be diabetic and more often labeled as having an unknown native kidney disease [Table 1].
CKD-MBD related symptoms
Table 2 shows that the most commonly reported symptoms were bone pain (33.5%), proximal muscle weakness (26.2%) and pruritus (25.5%); however 45% were asymptomatic. Three patients had fragility fractures (fracture neck of femur 2, vertebral fracture 1). There was no difference between Stage 4 and 5 CKD patients in the prevalence of CKD-MBD related symptoms.

Biochemical parameters
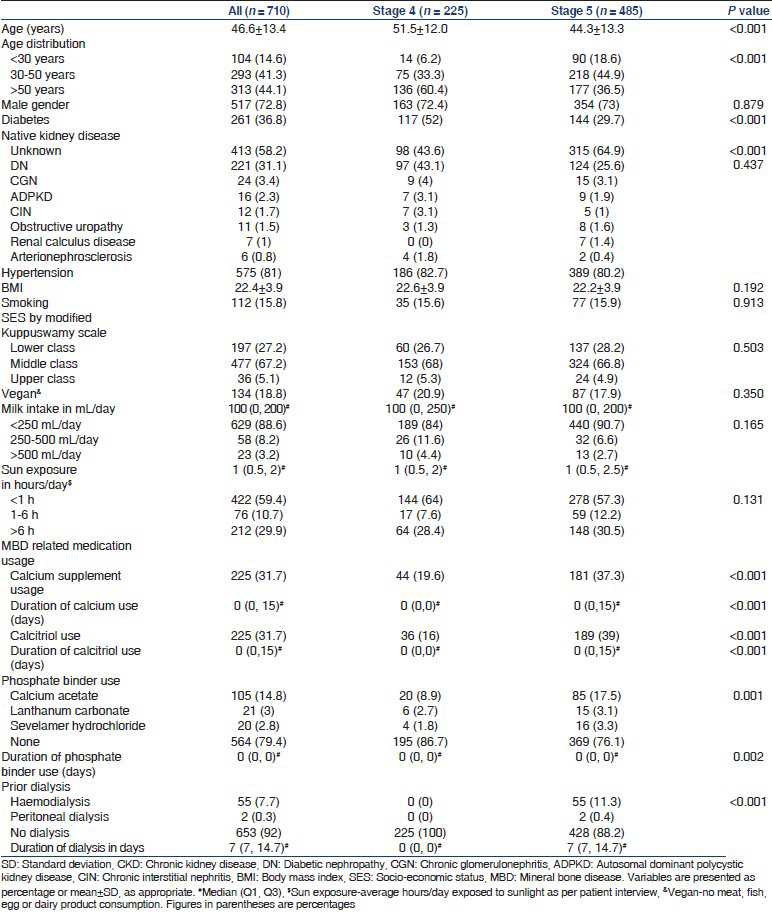
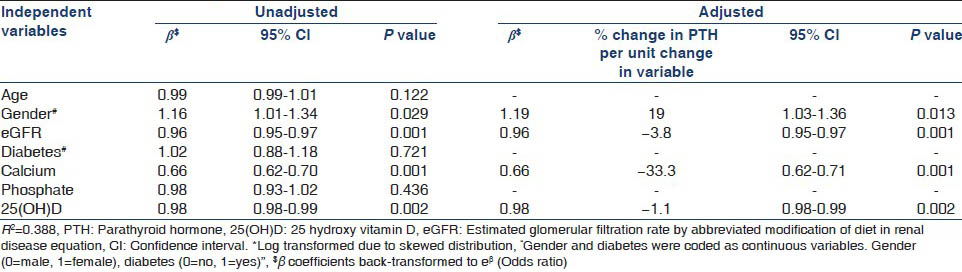
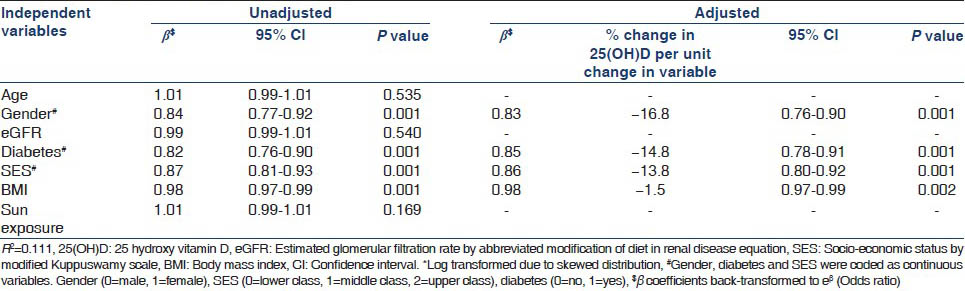
Biochemical parameters are summarized in Table 3. Patients with CKD Stage 5 had a lower serum calcium and higher serum phosphate and PTH levels. Alkaline phosphatase and 25(OH) D levels were not significantly different between CKD stages. Overall, 66.3% were hypocalcemic, 59% were hyperphosphatemic and 89.3% had 25(OH) D levels <30 ng/mL. PTH level ≥150 pg/mL was present in 57.3% in CKD Stage 4 and 89.5% in CKD Stage 5. Owing to skewed distribution, PTH and 25(OH) D were log transformed and multiple linear regression was used to determine their clinical and biochemical associations after adjusting for age, gender, diabetic status, socio-economic status, body mass index (BMI), sunlight exposure, eGFR, calcium, phosphate and 25(OH) D where appropriate. Table 4a and b show the clinical and biochemical variables affecting serum PTH and 25(OH) D. High PTH was associated with low eGFR, low serum calcium, low 25(OH) D levels and female gender. Low 25(OH) D levels were associated with female gender, diabetes, higher socio-economic status and higher BMI.

Valvular calcification
Echocardiographic evaluation was carried out in 471 patients. VC (calcification score ≥1), was present in 96%, and 51% had significant VC (calcification score ≥5). The mitral valve and posterior mitral annulus were the most common sites for calcification [Figure 2]. Mitral and aortic VC was present in 61.7%, 23.9% had only mitral VC, 2.7% had only aortic VC and only 11.5% had neither valve calcified. There was no difference in age, gender, diabetic status, calcium, phosphorus, PTH levels or eGFR between patients with isolated mitral and isolated aortic VC. The median cardiac calcification score and prevalence of significant calcification did not significantly differ between CKD Stage 4 and 5 [Table 5]. Table 6 compares clinical and biochemical parameters between patients with and without VC. On stepwise binary logistic regression, adjusting for age, gender, diabetic status, hypertensive status, smoking, eGFR, calcium, phosphate, PTH and 25(OH) D levels, patients with VC (calcification score ≥1) were found to be older (odds ratio [OR]: 1.04, 95% confidence interval [CI]: 1.01-1.08, P = 0.017) and had lower 25(OH) D levels (OR: 0.951, 95% CI: 0.91-0.99, P = 0.025) compared with patients without VC (calcification score = 0).
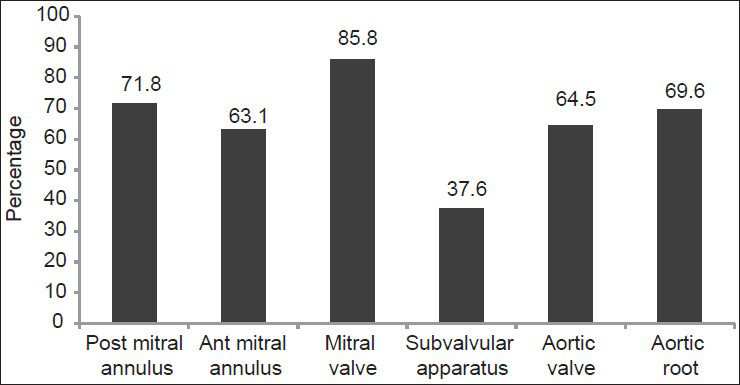
- Distribution of cardiac calcification. The percentage of patients with cardiac calcification at various sites as assessed by transthoracic echocardiography

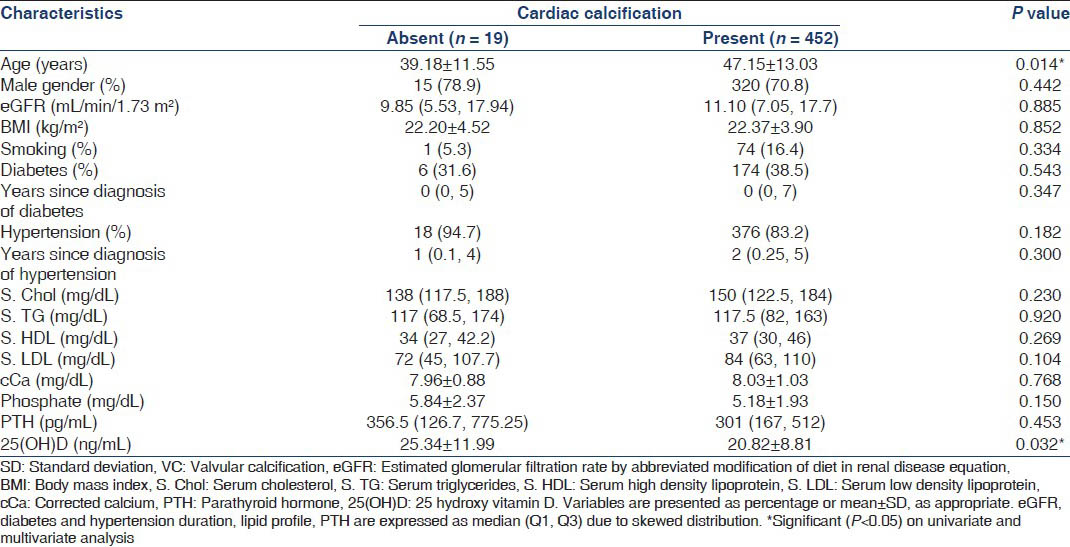
Abdominal aortic calcification
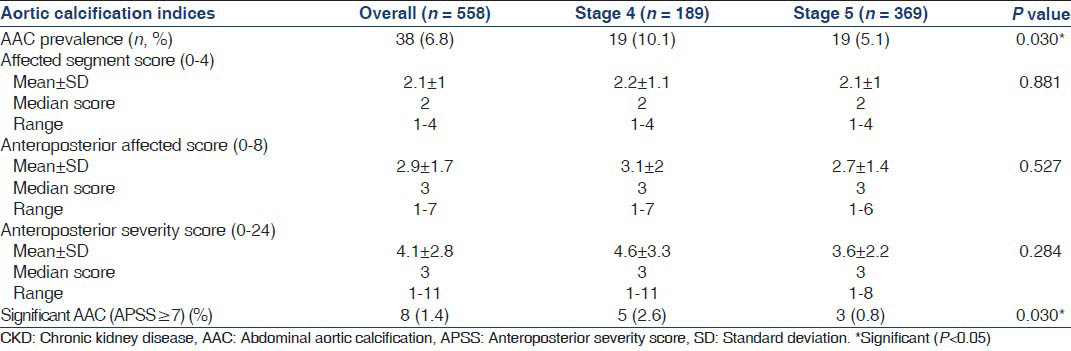
Lateral abdominal X-rays were available for 558 patients. AAC was seen in only 38 patients (6.8%), the most common site being the posterior aortic wall opposite L3 and L4 vertebrae [Figure 3]. Table 7 shows that Stage 4 CKD patients had a higher prevalence of AAC (10% vs. 5.1% P = 0.03) and significant AAC, defined as an anteroposterior segment score ≥7 (2.6% vs. 0.8%, P = 0.030). Table 8 compares clinical characteristics between patients with and without AAC. Patients with AAC were older, predominantly male, diabetic, hypertensive and smokers. They also had a higher eGFR and consequently a lower phosphorus level compared with those without AAC. Using multivariate binary logistic regression, after adjustment for age, gender, diabetic status, hypertensive status, eGFR and smoking, age was the only factor found to be independently associated with AAC (OR: 1.10, 95% CI: 1.05-1.15, P < 0.001). There was no association between the presence of AAC and VC.
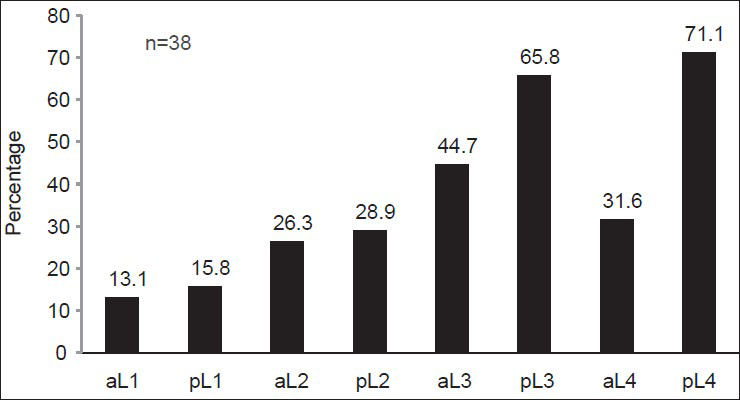
- Distribution of abdominal aortic calcification. The major sites of calcification among the 38 patients detected to have abdominal aortic calcification on X ray. On X axis, aortic segments are denoted as ‘a’ or ‘p’ referring to anterior or posterior aortic wall, followed by the lumbar vertebra opposite the aortic wall seg`ment. (eg. aL1 refers to the anterior aortic wall opposite the first lumbar vertebra)

Discussion
The prevalence of CKD in India has been estimated to range between 0.78% and 0.87%.[78] Despite the efforts of the CKD Registry of India, which collates data from an estimated 199 affiliated centers,[9] data regarding the characteristics of untreated CKD-MBD in pre-dialysis patients in India is scarce; a gap that this study specifically sought to bridge.
The study population comprised predominantly young and middle aged patients belonging to the low and middle income group, with males outnumbering females 3:1, which is representative of the referred CKD population in India.[9] In 45%, no CKD-MBD related symptom was found, reinforcing the fact that CKD-MBD is a clinically silent disease.
The high prevalence of 25(OH) D deficiency, hypocalcemia and hyperparathyroidism in this study is consistent with findings from previous hospital based surveys on CKD-MBD in India.[1011] Although not considered an essential component of CKD-MBD assessment in the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, 25(OH) D deficiency plays an important role in modifying and potentiating CKD-MBD in the Indian subcontinent and its ubiquitous presence has a deep rooted nutritional, genetic and socio-economic basis. This includes, low dietary intake of vitamin D, lack of a national vitamin D food fortification program,[12] increased activity of the 25(OH) D degrading enzyme 25(OH) D 24 hydroxylase in skin fibroblasts of Indians[13] and dark skin pigmentation requiring longer exposure to ultraviolet (UV) rays to achieve adequate 25(OH) D levels.[14] Patients of a higher socio-economic status, in whom 25(OH) D deficiency is more common,[15] reside in cities, where atmospheric pollution, cramped living spaces and sedentary occupation reduce their exposure to UV rays, compared with laborers and farmers who comprise the lower socio-economic strata.[16] They are also more likely to be obese, leading to sequestration of cutaneously synthesized and dietary vitamin D in adipose tissue and low bioavailability.[1718] Women in particular, have low 25(OH) D levels due to poor dietary intake, multiple unplanned, unspaced pregnancies and because 75% have less than 1 hour of sunlight exposure per day.[19] The higher prevalence of hyperparathyroidism in women is therefore a reflection of 25(OH) D deficiency. Diabetes, a national epidemic of sorts, is also associated with 25(OH) D deficiency as the latter has been shown to impair insulin secretion.[20]
VC on echocardiography was assessed using a global calcification scoring system described by Pressman et al.[6] The mitral valve and posterior mitral annulus were the most common sites of calcification in our study. While aortic VC increases with age and is considered a degenerative process, mitral annular calcification is more strongly associated with abnormalities in mineral metabolism.[21] However, this study did not demonstrate any difference in biochemical parameters between patients with isolated mitral or aortic VC.
Of the 471 patients assessed, 96% had calcification at one of eight valvular sites (i.e. calcification score of ≥1) and none had underlying rheumatic or intrinsic valvular disease. The prevalence of mitral VC (85.8%) and aortic VC (64.5%) in this study was much higher than that reported in Caucasian pre-dialysis CKD subjects (31% combined mitral and aortic VC) by Leskinen et al.[22] Ghosh et al., reported VC in 25% and 46% of Indian CKD Stage 4 and 5D patients respectively. In contrast, the prevalence of mitral and aortic VC in healthy Indian subjects aged 60-64 is only 2% and 28% respectively.[23] Thus, even prior to calcium and vitamin D analog supplementation, the prevalence of VC in young and middle aged CKD patients is significantly higher than that of healthy elderly Indian subjects, indicating that CKD itself promotes VC, as has been confirmed by studies in the West.[24] However, the higher prevalence of VC in Indian CKD patients compared with their Caucasian counterparts indicates that there may be geographic or genetic risk factors for VC unique to the Indian subcontinent, though this has not been studied either in the CKD or the general population. In this study, patients with VC were older and had lower 25(OH) D levels compared with those without VC. While the association of age with VC is in concordance with previously published data,[2225] Vitamin D has a U shaped relation with vascular calcification, with both low and high levels associated with an increased risk of extraskeletal calcification.[26] Vitamin D deficiency has been postulated to promote calcification through inflammatory cytokine release, decrease in calcification inhibitors such as fetuin-A and matrix Gla protein, and inhibition of klotho, one of whose roles is to prevent extraskeletal osteoblastic matrix deposition.[27] While the association between 25(OH) D deficiency and VC in this study does not prove causation, the nature and significance of this association requires further clarification through prospective longitudinal studies in Indian CKD patients. Aortic and mitral VC have been associated with a 1 year all-cause mortality of 57%, compared with 15% in patients with neither valve calcified,[28] and hence such widespread prevalence of VC even prior to dialysis initiation is a cause for concern.
AAC as assessed by lateral abdominal X-ray was found in 6.8% of the 558 patients surveyed. Lateral abdominal X-ray calcification score of ≥7 has sensitivity and specificity of 67% and 91% for coronary artery calcification (CAC) score of ≥100 Agatston units on electron beam computed tomography (EBCT).[29] Thus, despite a lower sensitivity, the presence of significant AAC on X-ray is strongly associated with a significant CAC and according to KDIGO guidelines,[3] can be used as a screening tool in the absence of EBCT.
Our study had a very low prevalence of AAC compared with studies involving Caucasian CKD patients,[30313233] which have reported a 49-90% prevalence on multislice computed tomography (MSCT) in Stage 3 and 5 CKD patients with a mean age of 58-71 years. Shantha et al., using a lateral abdominal X-ray for screening, found a prevalence of 76.9% in 26 Indian pre-dialysis Stage 5 CKD patients who had a mean age of 56.6, 65% of whom were receiving calcium containing phosphate binders.[34] We found AAC to be associated with traditional risk factors for atherosclerosis such as age, diabetes, hypertension, smoking and male sex, though only the association with age was found to be significant on adjusted analysis. Thus, AAC in pre-dialysis patients appears to be the result of calcification of atherosclerotic plaque as described in the general population. The low prevalence of AAC in this study is due to both the lower sensitivity of the screening modality and a younger study population (mean age 46.6 years) who had not received long-term calcium or vitamin D supplements.
The higher prevalence and greater severity of AAC in Stage 4 CKD patients is explained by the fact that Stage 4 CKD patients were older. Data from the CKD registry of India, also suggests that Stage 5 CKD patients in India are younger than patients in Stage 3 and 4 CKD at presentation,[9] which is an indirect indicator of the mortality associated with CKD in the elderly.[35] Since aortic calcification has been linked to all cause and cardiovascular mortality in the general[36] and dialysis population,[37] the presence of AAC by Stage 4 CKD may identify a subgroup of patients at high-risk for cardiovascular events who may benefit from more aggressive management of modifiable atherosclerotic risk equivalents.
This study has several strengths. This is the largest study to specifically assess untreated CKD-MBD in pre-dialysis Indian patients. The exclusion of patients who had received calcium supplements, phosphate binders or vitamin D analogs for >3 months allowed a more robust assessment of biochemical parameters at baseline. Ours is also the first study to use a semi-quantitative echocardiographic scoring system to assess global cardiac calcification burden in CKD patients. Although yet to be validated in CKD, the study from which this scoring system was derived[6] found a score of ≥5 to have a positive predictive value of 60% for a CAC score of ≥400 on MSCT and hence this scoring system can be used as a substitute for EBCT/MSCT in resource poor settings. Compared with most other CKD-MBD studies, which reported calcification at the mitral and/or aortic valve alone, the use of a calcification score assessing calcification at eight valvular sites increased the sensitivity for detection of VC and partly explains its high prevalence in the study subjects. Significant VC (score ≥5) was found in 51%, suggesting that in a majority, the calcification was likely to be of clinical significance.
The inherent limitation of a hospital based survey involving a referred patient population is that it cannot describe the epidemiology of CKD-MBD in the community. Although mean age, gender ratio, etiology of kidney disease, diabetic and socio-economic status of patients in this study is similar to the standard referred population described in the CKD registry of India,[9] population based surveys that have assessed the prevalence of CKD in India have shown the mean age of CKD patients to range from 52 to 59 years, 48-61% of whom are males.[738] Thus, a cross sectional survey of CKD-MBD in the community would have had an older patient cohort and a greater female representation than the present study. This in turn may have yielded a higher prevalence of 25(OH) D deficiency, hyperparathyroidism, VC and AAC than this study reported. Despite the potentiating effect of 25(OH) D deficiency on CKD-MBD as shown in this study, its measurement is not an essential component of CKD-MBD assessment as per the KDIGO guidelines.[3] In addition, 25(OH) D levels are influenced by a variety of environmental, socio-economic and genetic factors, already alluded to previously, which were not assessed in this study. Cardiac and aortic calcification could not be assessed in all subjects due to logistic reasons; however, baseline characteristics did not differ between those with and without these investigations. Bone histomorphometry for histological assessment of CKD-MBD and EBCT/MSCT to assess vascular calcification were not available at our center due to resource limitations. The lack of a sensitive imaging modality may partly explain the low prevalence of AAC in the study population. Lastly, although intriguing, the cross-sectional nature of the study design does not allow us to establish a direct causal relationship between 25(OH) D deficiency and the high prevalence of VC.
To conclude, this survey reveals a huge burden of hypocalcemia, vitamin D deficiency and VC in Indian CKD patients, well-established by the pre-dialysis stage. VC is associated with older age and vitamin D deficiency. AAC is associated with older age and is uncommon. The association between vitamin D deficiency and VC needs to be further clarified through experimental and prospective longitudinal studies.
Acknowledgment
We thank Prof. George T John for his patient and critical reading of the manuscript and for his mentorship during the study period and beyond.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third national health and nutrition examination survey. Am J Kidney Dis. 2003;41:1-12.
- [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1-130.
- [Google Scholar]
- Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519-30.
- [Google Scholar]
- New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis. 1997;132:245-50.
- [Google Scholar]
- Can total cardiac calcium predict the coronary calcium score? Int J Cardiol. 2011;146:202-6.
- [Google Scholar]
- Prevalence of chronic renal failure in adults in Delhi, India. Nephrol Dial Transplant. 2005;20:1638-42.
- [Google Scholar]
- Experience with a program for prevention of chronic renal failure in India. Kidney Int Suppl. 2005;94:S75-8.
- [Google Scholar]
- What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol. 2012;13:10.
- [Google Scholar]
- High prevalence of vitamin D deficiency in North Indian adults is exacerbated in those with chronic kidney disease. Nephrology (Carlton). 2009;14:345-9.
- [Google Scholar]
- The high prevalence of chronic kidney disease-mineral bone disorders: A hospital-based cross-sectional study. Indian J Nephrol. 2012;22:285-91.
- [Google Scholar]
- Vitamin D metabolism is altered in Asian Indians in the southern United States: A clinical research center study. J Clin Endocrinol Metab. 1998;83:169-73.
- [Google Scholar]
- Indian and Pakistani immigrants have the same capacity as Caucasians to produce vitamin D in response to ultraviolet irradiation. Am J Clin Nutr. 1986;44:683-5.
- [Google Scholar]
- Vitamin D status in Andhra Pradesh: A population based study. Indian J Med Res. 2008;127:211-8.
- [Google Scholar]
- Modern India and the vitamin D dilemma: Evidence for the need of a national food fortification program. Mol Nutr Food Res. 2010;54:1134-47.
- [Google Scholar]
- Vitamin D status of apparently healthy schoolgirls from two different socioeconomic strata in Delhi: Relation to nutrition and lifestyle. Br J Nutr. 2008;99:876-82.
- [Google Scholar]
- Bone status of Indian women from a low-income group and its relationship to the nutritional status. Osteoporos Int. 2005;16:1827-35.
- [Google Scholar]
- Vitamin D and diabetes: Let the sunshine in. Diabetes Educ. 2008;34:939-40. 942, 944 passim
- [Google Scholar]
- Cardiac valve calcification in haemodialysis patients: Role of calcium-phosphate metabolism. Nephrol Dial Transplant. 1998;13:2037-40.
- [Google Scholar]
- Valvular calcification and its relationship to atherosclerosis in chronic kidney disease. J Heart Valve Dis. 2009;18:429-38.
- [Google Scholar]
- Echocardiographic assessment of a healthy geriatric population. J Indian Acad Clin Med. 2004;5:47-51.
- [Google Scholar]
- Progression of coronary artery calcification in predialysis patients. Am J Nephrol. 2007;27:152-8.
- [Google Scholar]
- Relations of inflammation and novel risk factors to valvular calcification. Am J Cardiol. 2006;97:1502-5.
- [Google Scholar]
- Role of vitamin D in vascular calcification: Bad guy or good guy? Nephrol Dial Transplant. 2012;27:1704-7.
- [Google Scholar]
- Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: A prospective study. J Am Soc Nephrol. 2003;14:159-68.
- [Google Scholar]
- Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int. 2006;70:1623-8.
- [Google Scholar]
- Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381-7.
- [Google Scholar]
- Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant. 2006;21:707-14.
- [Google Scholar]
- Prevalence and associations of coronary artery calcification in patients with stages 3 to 5 CKD without cardiovascular disease. Am J Kidney Dis. 2008;52:849-58.
- [Google Scholar]
- Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant. 2008;23:586-93.
- [Google Scholar]
- Is abdominal aortic calcification score a cost-effective screening tool to predict atherosclerotic carotid plaque and cardiac valvular calcification in patients with end-stage renal disease? Indian J Nephrol. 2012;22:431-7.
- [Google Scholar]
- Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA. 2005;293:1737-45.
- [Google Scholar]
- Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529-34.
- [Google Scholar]
- Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49:417-25.
- [Google Scholar]
- Epidemiology and risk factors of chronic kidney disease in India-results from the SEEK (Screening and early evaluation of kidney disease) study. BMC Nephrol. 2013;14:114.
- [Google Scholar]







