Translate this page into:
Reassessing Metformin’s Potential in Autosomal Dominant Polycystic Kidney Disease (ADPKD): A Call for Further Research
Corresponding author: Jasmine Sethi, Department of Nephrology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. E-mail: jasmine227021@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sethi J. Reassessing Metformin’s Potential in Autosomal Dominant Polycystic Kidney Disease (ADPKD): A Call for Further Research. Indian J Nephrol. 2025;35:444-5. doi: 10.25259/IJN_542_2024
Dear Editor,
I want to express our sincere gratitude for the thoughtful and constructive letter regarding our recent study.1 In response to the letter, we intend to explore the factors that may have contributed to the nonsignificant findings of our study.
We emphasize that our study was primarily a feasibility assessment and did not have sufficient power or duration to draw definitive conclusions about the metformin efficacy. Due to the limited duration, we were unable to demonstrate a significant reduction in glomerular filtration rate (GFR) with metformin. The use of estimated GFR (eGFR) as an endpoint in participants with well-preserved eGFR requires large trials of long duration because of the slow progression of eGFR in the early stages of autosomal dominant polycystic kidney disease (ADPKD). Alternative clinical trial end points like biomarkers (copeptin) or prognostic enrichment through imaging/genetics can be employed to detect benefit at an earlier stage. Peronne et al. calculated the ideal sample size as 700–800 study participants and four to five years of study duration in order to detect a 25% improvement in eGFR decline and 45% reduction in height-adjusted total kidney volume (htTKV) slope.2
Lack of metformin for a meaningful beneficial effect on eGFR declines and htTKV could also be explained by the fact that only around half of the patients could tolerate the maximal tolerated dose in our study. This was consistent with the trial of administration of metformin in polycystic kidney disease (TAME-PKD) and Brosnahan et al. where compliance to full dose (2 gm/day) metformin was seen in around 50–65% participants2,3 [Figure 1]. As rightly mentioned by the author, further trials using personalized metformin dosing or extended release (XR) metformin formulations should be planned for better patient adherence. We believe that given its potential benefits, metformin could be a promising therapeutic option for ADPKD and should be evaluated in larger clinical trials.
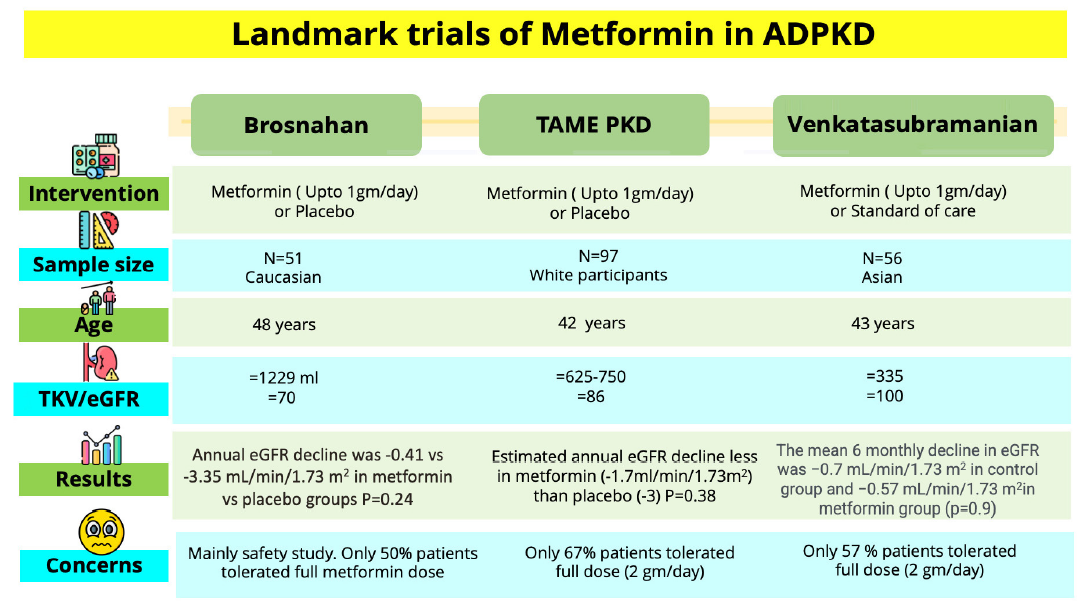
- Existing metformin studies in ADPKD. ADPKD: Autosomal dominant polycystic kidney disease, TAME PKD: Trial of administration of metformin in polycystic kidney disease, eGFR: estimated glomerular filtration rate, TKV: Total kidney volume.
Conflicts of interest
There are no conflicts of interest.
References
- Metformin versus standard of care in patients with autosomal dominant polycystic kidney disease – A Randomized control trial. Indian J Nephrol. 2025;35:410-6.
- [Google Scholar]
- Primary results of the randomized trial of metformin administration in polycystic kidney disease (TAME PKD) Kidney Int. 2021;100:684-96.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Metformin therapy in autosomal dominant polycystic kidney disease: A feasibility study. Am J Kidney Dis. 2022;79:518-26.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






