Translate this page into:
Recent Updates in the Diagnosis and Management of Kidney Diseases in Multiple Myeloma
Corresponding author: Priti Meena, Department of Nephrology, All India Institute Medical Sciences, Bhubaneswar, India. E-mail: pritimn@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jhaveri KD, Meena P, Bharati J, Bathini S. Recent Updates in the Diagnosis and Management of Kidney Diseases in Multiple Myeloma. Indian J Nephrol. 2025;35:8-20. doi: 10.25259/ijn_491_23
Abstract
Multiple myeloma (MM) represents a difficult-to-treat plasma cell malignancy and the second most common hematologic malignancy in adults, significantly impacting kidney function. The spectrum of kidney involvement in MM is broad, encompassing electrolyte imbalances, tubular injury, and even rare glomerular diseases. The evolution of MM treatment modalities has led to notable improvements in the long-term survival of patients experiencing kidney-related complications. Over the past decade, groundbreaking therapeutic agents have emerged, including proteasome inhibitors, immunomodulatory drugs, anti-CD38 monoclonal antibodies, selective inhibitors of nuclear export, and antibody-drug conjugates. These novel therapies have revolutionized the landscape of MM management, offering new hope for patients and challenging the traditional treatment paradigms. This comprehensive review explores recent advances in the diagnosis and management of MM, emphasizing the pivotal role of these innovative therapeutic agents in improving patient outcomes. We delve into the intricacies of diagnosing MM, highlighting the significance of early detection and precise diagnostic tools. We elucidate the evolving treatment strategies, emphasizing the mechanisms of action and clinical efficacy of the latest agents. This manuscript provides valuable insights into the ever-evolving field of MM management, shedding light on the remarkable progress achieved in enhancing the prognosis and quality of life of MM patients.
Keywords
Glomerular diseases
Myeloma cast nephropathy
Multiple myeloma
Plasma cell disorders
Proteasome inhibitors
Introduction
Multiple myeloma (MM) is a hematologic neoplasm of plasma cells (PC) with a myriad of clinical manifestations, ranging from incidental findings to multiple organ failures. It accounts for 1% of all malignancies and contributes to 10% of hematologic cancers.1 MM almost always evolves from an asymptomatic stage of monoclonal gammopathy of undetermined significance (MGUS), with gradual progression to an overt MM.2 In recent years, significant insights into this disease’s pathophysiology and therapeutic targets have emerged.
Though overall survival has improved over the past few years, it remains at just over 5 years with most survivors suffering relapses, using four or more lines of therapy.3 MM has a heterogeneous presentation, varying in organ involvement. The disease usually has a sub-acute onset. The commonest clinical manifestations include anemia, bony pains, asymptomatic rise in serum creatinine, fatigue, and hypercalcemia. Figure 1 shows various organ involvement and clinical presentation in MM.4 This review discuss the changing horizons of myeloma and kidney disease management and provide future ideas for research.
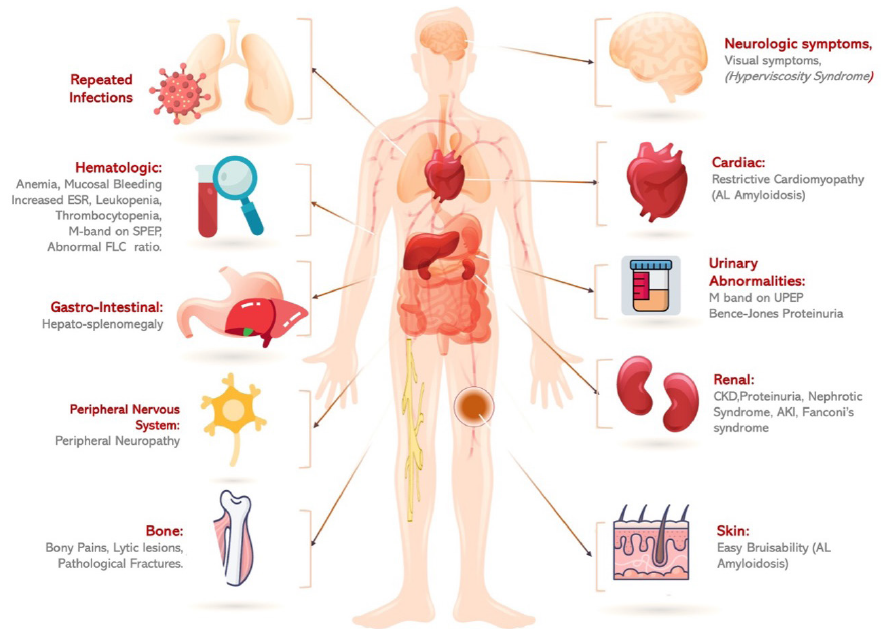
- Various organ manifestations in multiple myeloma. SPEP: Serum protein electrophoresis, UPEP: Urine protein electrophoresis, FLC: Free light chains, CKD: Chronic kidney disease, ESR: Erythrocyte sedimentation rate, AL: Amyloid light chain, AKI: Acute kidney injury, GN: Glomerulonephritis.
Kidney dysfunction in MM
The kidney manifestations in MM range from asymptomatic proteinuria, unexplained chronic kidney damage (CKD), nephrotic syndrome, and Fanconi’s syndrome to acute kidney injury (AKI) [Table 1 and Figure 2]. About 50% of patients with MM experience AKI or CKD at some point during their illness, with 10% requiring dialysis upon initial diagnosis of MM.5,6 Figure 3 shows the causes of kidney dysfunction in MM patients.
| Nephrotic syndrome | Nephrotic syndrome is a more predominant finding in AL amyloidosis, C3 GN, immunotactoid GN, cryoglobulinemic GN, MIDD, or PGMID. In AL amyloidosis, around 50% of patients present with asymptomatic proteinuria or nephrotic syndrome. |
| AKI/subacute kidney injury | AKI can be present in up to half of MM patients. It could be due to mIg-dependent or mIg-independent factors. |
| CKD | A steady or gradual rise in serum creatinine levels is frequently observed in MM patients. CKD might be the primary manifestation in patients with immunoglobulin light chain (AL) amyloidosis, MIDD, or LCPT. Patients who have had one or more episodes of LCCN with incomplete recovery of kidney functions can progress to CKD. Other factors, like hypertension or diabetes mellitus, may contribute to CKD development in MM patients. |
| Electrolyte abnormality | Electrolyte imbalances such as hypercalcemia, hyponatremia, and Fanconi syndrome are frequently observed in MM. Fanconi syndrome is typically suspected if hypokalemia, metabolic acidosis, and hypophosphatemia are present. Because proximal tubules are predominantly affected by the reabsorption and accumulation of light chains, tubular dysfunction, and Fanconi syndrome are commonly experienced in LCPT. The monoclonal LC responsible for Fanconi syndrome have a variable domain with unique biochemical properties, rendering them resistant to lysosomal degradation in tubular cells. Anti-myeloma drugs like lenalidomide can also cause hypokalemia and metabolic acidosis. |
| Specific disorders and presentation | |
| LCCN | LCCN usually presents with AKI and proteinuria and is also considered a MDE. |
| PGNMID | PGNMID mimics immune-complex glomerulonephritis and the usual presentations are nephrotic syndrome, hematuria, and kidney dysfunction. Hypertension is a common feature. |
| MIDD | Patients with MIDD can have both proteinuria and kidney dysfunction in addition to light chain excretion with associated cast nephropathy. MIDD usually precedes the diagnosis of dysproteinemia. |
| Light chain crystalline podocytopathy | Light chains (usually kappa) crystallize inside podocytes, leading to symptoms such as proteinuria and CKD. |
| CSH | Characterized by an accumulation of light chain crystals in histiocytes that congregate in extramedullary locations such as the kidney. In kidneys, the histiocytes containing light chain or immunoglobulin inclusions can be found in the interstitium and glomeruli. |
CKD: Chronic kidney damage, AKI: Acute kidney injury, CSH: Crystal-storing histiocytosis, GN: Glomerulonephritis, MIDD: Monoclonal immunoglobulin deposition diseases, PGNMID: Proliferative glomerulonephritis with monoclonal IgG deposits, LCPT: Light chain proximal tubulopathy GN, LCCN: Light chain cast nephropathy, MDE: Myeloma defining event.
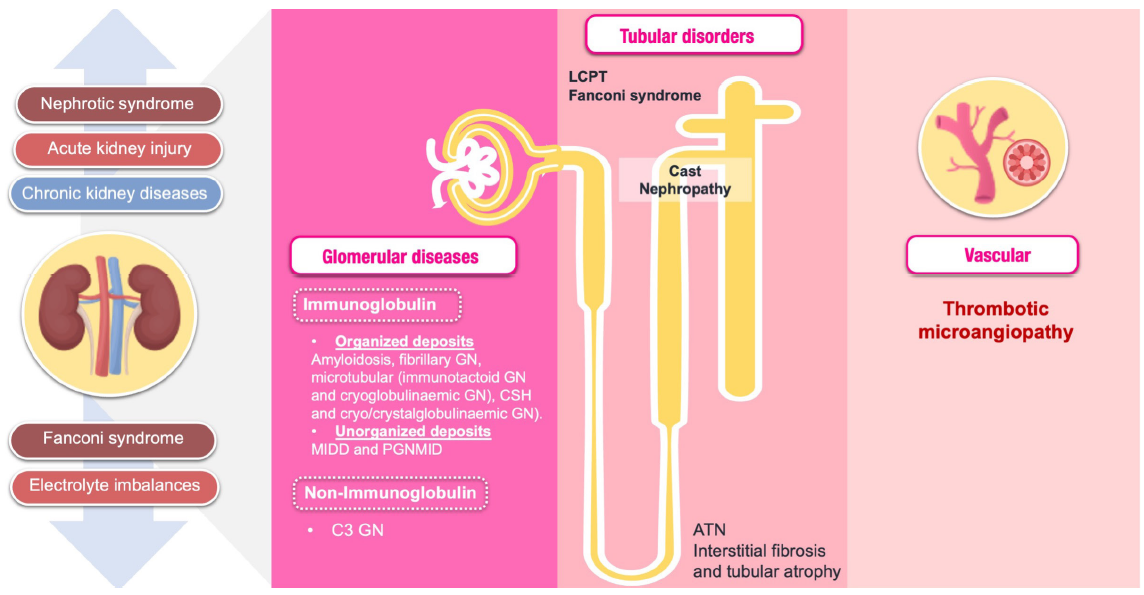
- Spectrum of kidney involvement in multiple myeloma. ATN: Acute tubular necrosis, CSH: Crystal-storing histiocytosis, LCPT: Light chain proximal tubulopathy, GN: Glomerulonephritis, MIDD: Monoclonal immunoglobulin deposition diseases, PGNMID: Proliferative glomerulonephritis with monoclonal IgG deposits.
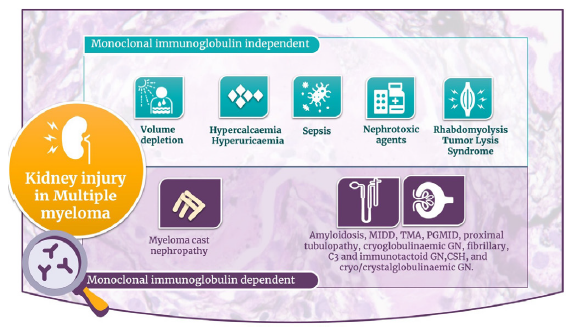
- Causes of kidney injury in multiple myeloma. CSH: Crystal-storing histiocytosis, GN: Glomerulonephritis, MIDD: Monoclonal immunoglobulin deposition diseases, PGNMID: Proliferative glomerulonephritis with monoclonal IgG deposits, TMA: Thrombotic microangiopathy.
Evaluation
According to the revised International Myeloma Working Group (IMWG) criteria,7 the diagnosis of MM requires the presence of one or more myeloma-defining events (MDE) with evidence of either ≥10% clonal bone marrow plasma cells (cBMPCs) or plasmacytoma (biopsy-proven or extramedullary) or evidence of the presence of cBMPCs ≥60% or serum free light chain (FLC) ratio ≥100 (provided involved FLC level is ≥100 mg/L), and more than one focal lesion on magnetic resonance imaging (MRI). The myeloma-defining events are shown in Figure 4.
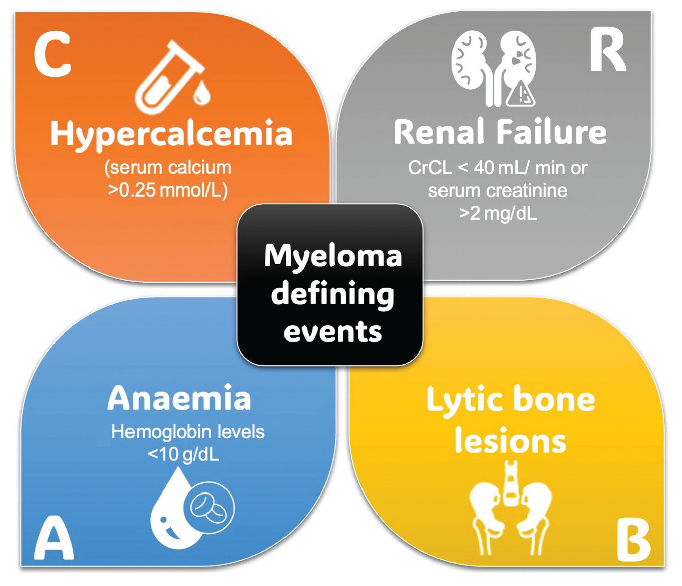
- Myeloma defining events. Crcl: Creatinine clearance.
Serum protein electrophoresis (SPEP) and serum immunofixation (IFx)
The serum protein electrophoresis (SPEP) is commonly used to screen for the presence of monoclonal protein (M protein).8–10 M protein is described as monoclonal immunoglobulin (mIg) produced by monoclonal proliferation of PC.11,12 In recent times, capillary zone electrophoresis has gained popularity in clinical usage where it offers an automated methodology for sample processing.13 Furthermore, the utilization of higher voltage applications results in minimal band broadening.14 Daratumumab, a therapeutic monoclonal antibody (t-mAb) used in the treatment of MM, has been observed to interfere with the standard SPEP.15 The evaluation of electrophoretic response becomes increasingly challenging when multiple t-mAbs are being used in a single patient. The differentiation between the M-protein and t-mAbs can be achieved using a double hydrashift assay.16 In their analysis, Liu et al. support the feasibility of utilizing the antigen-specific t-mAbs depletion assay as a means of eliminating t-mAb from patient sera, irrespective of the platform employed.17
Serum immunofixation is considered the gold standard and is recommended for the confirmation of the presence of the M band in serum.7,18–22 The utilization of IFx enhances the sensitivity of SPEP by approximately 10 times. In cases with a strong suspicion of MM when SPEP is negative, IFx should be performed. The main characteristics of SPEP and IFx are provided in Table 2.
| Technique | Method | Characteristic | Remark |
|---|---|---|---|
| Serum electrophoresis |
Screening procedure. Separates serum proteins based on their physical properties such as size and charge. |
M-spike is a distinct sharp band. In MM pts, M-spike typically exceeds 3 g/dL.10 In polyclonal gammopathy, a diffuse band displaying one or more heavy chains and light chains (kappa and lambda) is seen.9 Biclonal gammopathy is characterized by the occurrence of dual M-proteins. |
Up to one-fifth of MM cases can have an M protein spike of less than 1 g/dL. A small proportion (∼2%) of patients present with a true non-secretory form of MM.11 Some patients exhibit the production of only monoclonal LC. Typically, these chains are present in quantities that are insufficient to manifest as a discernible spike in the agarose gel, owing to their prompt elimination in the urine. M-proteins can migrate in the β fraction of the protein electrophoresis12 (approximately 30% of all IgAs may be concealed under the peak. IgD myeloma cases are relatively uncommon, and the M-protein may appear minute or entirely indiscernible). |
| Immunofixation |
Operates on the basis of dissociation of electrophoresis and the utilization of mIg high specificity (antigen-antibody reaction) and sensitivity to identify M components present in serum.22 Gold standard for the confirmation of the presence of the M band in serum. |
Characterizes M-protein subtypes identified by SPEP. Enables detection of minimal bands of 50–100 mg/L in serum and as low as 5 mg/L in urine.18 |
Detection of a minor M-protein in individuals diagnosed with AL, solitary plasmacytoma, extramedullary plasmacytoma, HCDD, LCDD, or following successful management of MM. A narrow M-protein can sometimes be obscured within the typical B or C regions, thereby being disregarded. Discrete band or spike manifestation is frequently not evident in HCDD. M-proteins of IgD and IgE types usually appear small in size and may be overlooked. |
M: Monoclonal spike; LC: Light chains; SPEP: Serum electrophoresis; AL: Amyloidosis; HCDD: Heavy-chain deposition disease; LCDD: Light chain deposition disease; MM: Multiple myeloma.
Urinary studies
A subset of patients (10% to 25%), exhibit an absence of detectable M spikes in SPEP.9 Detecting light-chain-only MM (LCMM) through SPEP is challenging, while urine electrophoresis (UPEP) may be utilized for this purpose. The recommended method for diagnosis and monitoring of LCMM is 24-hour urinalysis.7 Nevertheless, in most cases, Bence Jones proteins are detected in the urine by the presence of well-defined, distinct bands on urine UPEP. However, there are limitations when it comes to quantifying M-proteins in urine as the results can be influenced by impaired kidney function. To overcome the proximal reabsorption of the tubule, the serum levels of kappa FLCs must exceed 130 mg/L and lambda FLCs to exceed 278 mg/L, for detecting M-proteins in the urine.23
Upon detection, it is essential to confirm the monoclonality through urine IFx.24 It is recommended that individuals who have been diagnosed with plasma cell disorder (PCD) undergo a baseline UPEP, along with IFx, using a sample obtained from a 24-hour urine collection. In cases where IFx fails to demonstrate a monoclonal LC but UPEP reveals a localized globulin band, it is advisable to consider the possibility of heavy-chain deposition disease (HCDD).22 The assessment and documentation of a complete response in patients undergoing therapy also necessitate the subsequent use of urine IFx.
Free light chain measurement
In healthy individuals, the median serum lambda level is 7.3 mg/L (with a 95% range of 3.3–19.4 mg/L), the median kappa level is 12.7 mg/L (with a 95% range of 5.7–26.3 mg/L), and the ratio of kappa to lambda FLCs is 0.26 to 1.65.25,26 The kidneys metabolize and clear both kappa and lambda FLC. The half-life of monomeric lambda LC is approximately 2–4 hours, whereas dimeric kappa light chains, which have a larger molecular size, are eliminated from the body at a slightly slower rate of 3–6 hours. In cases of advanced kidney failure, the serum FLC half-life is raised to a range of 2–3 days.27 Increasing the reference range from 0.31 to 3.1 enhances specificity from 93% to 99% with similar sensitivity.28,29 Kidney dysfunction alone is unlikely to cause kappa/lambda ratios >3.0.30,31 The immunoassays for serum FLC involve the use of polyclonal anti-free LC antibodies conjugated with latex. These antibodies exhibit a high level of specificity and affinity for serum-FLC.
Initially, after integrating FLC measurement in the diagnostic assessment of MM, only one assay technique utilizing polyclonal antibodies (Freelite) was available for the quantification of FLC.32 Following this, two further assays have been developed: the N Latex FLC assay, which utilizes monoclonal antibodies, and the Sebia FLC assay, which employs polyclonal antibodies and is based on ELISA technology.33,34 The currently available clinical trial data and recommendations have been solely predicated on outcomes derived from the Freelite assay. According to a recent study, there were noticeable inconsistencies in the involved FLC/uninvolved FLC ratio across various assays.35 Therefore, it is not suggested to utilize these assays interchangeably when monitoring patients.
The IWMG has proposed a serum panel for the assessment of monoclonal PCD that includes serum FLC, SPEP, and serum IFx.7 It applies to all patients except those with AL amyloidosis, for whom a 24-hour urine IFx remains necessary. The sensitivity for MM detection is about 90% when serum protein IFx is done along with SPEP. If additional tests such as the serum FLC assay or urine M protein studies (UPEP and urine IFx) are further employed the sensitivity increases to 97% or higher.7,22,25
Utility of mass spectrometry (MS)
The possibility of non-invasive methods of diagnosing MM that compete with a biopsy is alluring but still reflects exploratory research.36,37 Mass spectrometry (MS) with intact matrix-assisted laser desorption/ionization time-of-flight method has been suggested by IWMG as a viable substitute for IFx in both clinical practice and clinical trials. Table 3 provides new and emerging tools for diagnosing and monitoring treatment for MM patients.
Treatment
The first-line treatments for myeloma cast nephropathy (MCN) and common types of Monoclonal Gammopathy of Renal Significance (MGRS), i.e., light chain deposition disease (LCDD) and light chain (AL) amyloidosis are similar, except that the level of kidney function and feasibility of kidney transplantation (KT) play dominant roles in deciding treatment in MGRS, as summarized in Figure 5.38 MM-related kidney impairment is a medical emergency that requires immediate intervention. Supportive therapy is an essential component of therapy in those with kidney impairment and is composed of hydration, cardiovascular monitoring, and maintaining calcium balance. Individuals with fluid depletion secondary to hypercalcemia need hydration (at least 3 L/day or 2 L/m2 per day). Patients with cardiac conditions require vigilant monitoring of fluid balance. Fluid challenge is appropriate for anuric patients. The evidence on restoring kidney damage with urine alkalization has not been well established.
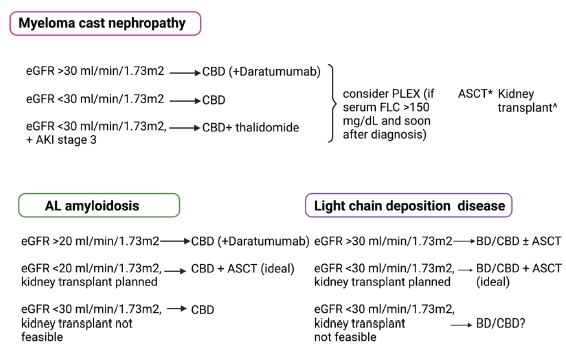
- First-line treatment of myeloma cast nephropathy, AL amyloidosis, and light chain deposition disease. CBD is C: Cyclophosphamide, B: Bortezomib, D: Dexamethasone; ASCT: Autologous stem cell transplantation, eGFR: Estimated glomerular filtration rate, AKI: Acute kidney injury, FLC: Free light chains, PLEX: Plasma pharesis, *ASCT is performed after hematological complete or very good partial response to achieve prolonged remission, ^kidney transplant can be performed if standard criteria for kidney transplantation are met, and complete hematological response is achieved.
Hematological response criteria are standardized in the treatment of MM.39 The kidney response in MM is defined by two criteria by the IMWG: (a) dialysis dependence and (b) a decrease in serum creatinine by 50% from baseline.38,40 Proteinuria reduction is an essential criterion in assessing treatment response in MGRS predominantly involving glomeruli like AL amyloidosis.41 A 30% or more reduction in proteinuria or proteinuria of 500 mg/day, along with no evidence of eGFR decrease to 25% from baseline, was validated as a kidney response criterion in a study on AL amyloidosis.41 Hematological remission correlates with kidney response in most but not all patients with myeloma cast nephropathy (MCN). Chronicity marker (interstitial fibrosis/tubular atrophy), the extent of cast formation, and age, besides hematological response, predict kidney response at follow-up.42 Kidney response correlates with overall survival in patients with MCN, independent of hematological response.
Myeloma cast nephropathy (MCN): There has been significant progress in treating MM recently. AKI at the time of diagnosis and non-recovery of AKI is associated with poor survival during the initial treatment.43 However, the resolution of AKI with bortezomib (needs no kidney dose modification) reverses the negative impact of AKI on long-term patient survival. In the phase III Hovon-65/HMMG-HD4 trial, replacing vincristine with bortezomib in the regimen containing adriamycin (A) and dexamethasone (D) led to significant improvement in overall survival in patients with serum creatinine 2 mg/dL (34% in the vincristine arm vs. 74% in the bortezomib arm) at 3 years.44 Overall survival and progression-free survival were similar in patients with and without serum creatinine 2 mg/dL in the bortezomib/adriamycin/dexamethasone arm. The bortezomib-based regimen is associated with a hematological overall response rate of 75%–80% and also an improved kidney overall response rate significantly. A combination regimen comprising bortezomib (commonly referred to as “V” for Velcade) and dexamethasone (D), with or without cyclophosphamide (C), is a well-established first-line therapy to treat MM patients with any degree of kidney injury, including estimated GFR <30 mL/min/1.73 m2.
No superiority was observed for cyclophosphamide/bortezomib/dexamethasone (kidney overall response rate: 51.1%) over bortezomib/dexamethasone (kidney overall response rate: 44.6%) regimen for treating newly diagnosed MM patients with AKI. However, patients with AKI stage 3 tended to do better with the cyclophosphamide/bortezomib/dexamethasone regimen.45 The immunomodulators are variably cleared by the kidneys and require dose adjustments in the presence of kidney dysfunction. A newer generation proteasome inhibitor, Carfilzomib, is associated with nephrotoxicity (thrombotic microangiopathy), and, therefore, not favored upfront.
Thalidomide (T) is minimally cleared by the kidneys; however, frequent side effects hamper administration. Lenalidomide (commonly referred to as “R” for Revlimid) is predominantly used to treat relapsed or refractory MM patients.46 Lenalidomide had similar effectiveness in relapsed/refractory MM patients with creatinine clearance < or >30 mL/min.47 However, the response was poor among patients on dialysis.
Pomalidomide is a newer immunomodulator indicated for refractory MM, failing at least two therapies, including lenalidomide and bortezomib. Pomalidomide is used at 4 mg daily for 1–21 days, in a 28-day cycle in all patients irrespective of kidney dysfunction.48 In a phase II trial, pomalidomide plus low-dose dexamethasone was efficacious in patients with eGFR <45 mL/min/1.73 m2; however, patients on dialysis had a poor response and more side effects.49
Adding daratumumab, an anti-CD38 monoclonal antibody, as an upfront therapy improved remission rates in MM patients. It has direct antitumor (clonal plasma cell) and immunomodulatory action. Phase III trials have observed better efficacy of daratumumab added to bortezomib/dexamethasone/thalidomide or lenalidomide compared to conventional regimens.50 Although these trials have excluded patients with severe kidney dysfunction, i.e., eGFR <30 mL/min, an increasing number of case reports describe the successful use of daratumumab in them.51
Role of extracorporeal therapies in treating light chain cast nephropathy (LCCN)
Serum-FLC levels correlate with the development of AKI and recovery of kidney function.52 A rapid, early, and significant reduction of serum-FLC levels after treatment is associated with the improvement of MCN.40,53 Rapid and effective removal of serum FLCs will likely reduce the exposure of non-involved tubules to the toxic effects of light chains and help in the recovery of kidney dysfunction as involved tubules regenerate in the absence of continued exposure to FLCs.53 Table 4 lists the existing evidence, from randomized controlled trials on the use of plasma exchange in MCN.
Given the trend of a benefit of plasmapheresis (PLEX) in severe MCN, and a theoretical possibility of effective removal of serum FLC, PLEX is being used in the initial management of severe AKI due to MCN, especially in the presence of serum FLC >150 mg/dL, to target; <50% reduction in serum FLC rapidly, at many centers.54 While some use high cut-off membranes, others have experimented with online high-efficiency hemodiafiltration for serum FLC removal.55
Autologous stem cell transplantation (ASCT): ASCT is a crucial component in the treatment of MM for eligible patients. ASCT is associated with greater hematological response (>70%) and more prolonged remission.56 Patients who achieve complete hematological response are considered for ASCT for deeper response and long-term progression-free survival.57 Patients should have good performance status and no cardiac contraindication to be fit for ASCT, as transplant-related mortality is otherwise significant.58 ASCT is a critical consideration in high-risk MM patients, who generally exhibit suboptimal responses to conventional treatment modalities. The cautionary stance extends to patients with kidney dysfunction, wherein ASCT necessitates careful evaluation. For patients with serum creatinine levels more than 2 mg/dL, adjustments to the conditioning regimen, including melphalan, are essential. A retrospective analysis of 370 MM patients undergoing ASCT revealed no significant differences in ASCT-related mortality, progression-free survival, or overall survival among those with varying degrees of kidney impairment or normal kidney function.59 In a separate study on ASCT for MM in patients with severe kidney impairment, 34 out of 35 achieved post-ASCT dialysis independence. There was no difference in 5-year progression-free survival and overall survival rates among patients with normal kidney function and severe kidney impairment.60 These findings highlight the safety and efficacy of ASCT as a viable therapeutic option for MM patients with CKD, including those dependent on dialysis.
In a subsequent analysis of 11 KT patients, eight of them underwent ASCT before KT showed hematologic progression in 75% of cases, with 45.5% experiencing death with functioning allografts at a median follow-up of 40 months. The 5-year graft survival was 66%.61
Kidney transplant (KT): KT is increasingly performed in patients with MM who achieve hematological control.61–63 Patients are treated with ASCT to achieve a profound and durable hematological response and then are taken up for KT.64 High-dose melphalan (dose modified as per kidney function) and ASCT is performed after about 6 to 12 months after starting chemotherapy, and KT is performed a further around 6 to 12 months after ASCT. Better control of plasma cell clones, use of maintenance chemotherapy after KT, prophylactic antibiotics, and supportive therapies for comorbidities have improved the success rate of KT in MM.58 Immunomodulatory agents like lenalidomide and thalidomide are avoided due to the associated risk of allograft rejection. In a small study, 40% of patients had an MM relapse within 2 years of KT despite ASCT and complete hematological response before the KT.64 Without an MM relapse, all patients were alive at a median of 55 months. High-risk (genetic risk) MM tends to relapse early and may not be a good choice for KT.65 Novel risk stratification scoring systems may improve the prediction of early relapse in MM patients.6 Until a standardized system is established, patients with high-risk MM who have persistent minimal residual disease (MRD) positive are best not offered KT.66,67
Monoclonal Gammopathy of Renal Significance (MGRS)
Patients with MGRS are treated like MM with clone-directed therapy, including ASCT. While KT is feasible in most cases with monoclonal immunoglobulin deposition disease (MIDD) and proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID) having isolated kidney involvement, patients with AL amyloidosis are often morbid with cardiac/liver involvement and deemed unfit for ASCT and KT. Nevertheless, as hematological response improved significantly with recent chemotherapy and ASCT, more patients are now needing kidney replacement therapy.68 As the glomeruli are invariably involved by MGRS, the kidney response criterion in evaluating patients with MGRS includes 24-hour proteinuria.
While it is known that kidney response might lag behind the hematological response in AL amyloidosis, the decision to switch to second-line therapy should be quick if either hematological or organ response is not achieved.69,70 In a retrospective multicenter study of 237 patients with AL amyloidosis (without MM), high-dose chemotherapy/ASCT was used as a first-line therapy in 31% of patients.70 Daratumumab combined with a conventional regimen of bortezomib, cyclophosphamide, and dexamethasone was superior to the conventional regimen in patients with newly diagnosed AL amyloidosis in terms of complete hematologic response and survival free from major organ deterioration in the ANDROMEDA trial.71 More patients had kidney response at 6 months in the daratumumab arm (53%) than in the control arm (24%). Daratumumab has already been used successfully in treating refractory AL amyloidosis.72,73
Kidney transplant was observed to have improved overall patient survival by 10.5 years,68 8.6 years,70 and 10.3 years,74 especially those who achieved a complete or very good partial response after chemotherapy versus those with partial response or non-response. Most patients were treated with chemotherapy and ASCT before KT; however, 15% were treated with ASCT after KT without a difference in patient or graft survival in the two groups. Most patients selected for KT did not have advanced extrarenal organ involvement, Mayo stage 2 or more cardiac involvement, underwent ASCT, and had complete/very good partial response (77%).70 Therefore, patients reported in these retrospective studies represent only the selected ones with the best outcome to therapies for AL amyloidosis, not the whole AL amyloidosis patient population.
The median time between hematological response and KT was 2.2 years in the largest multicenter study.70 Recurrence of amyloidosis in the kidney developed in 29% of patients; however, the successful hematological treatment prevented graft loss in 87% of patients. The wait time between hematological response and KT and the selection of anti-rejection therapies post-KT are not standardized with a guideline.
Successful reduction of serum FLC is associated with favorable kidney response in MIDD.75–79 Patients should be treated aggressively (high-dose chemotherapy and ASCT) to achieve the best hematological response before a KT, reducing the likelihood of recurrence after a kidney transplant.80 However, small B-cell/PC clones may not be curable with chemotherapy. Therefore, the timing of kidney KT in patients with MIDD is individualized. A KT can be performed without a complete hematological response in young patients without extrarenal organ involvement, good performance status, and immediate or nearing need for chronic dialysis. These patients can then be taken up for high-dose chemotherapy and ASCT for the best hematological response after KT.
Addition of daratumumab reduced serum FLC levels in patients with LCDD refractory to bortezomib/cyclophosphamide/dexamethasone.81 In a recent study of eight patients with LCDD refractory to bortezomib-based therapies and all having high PC clone burden (median serum FLC of 210 mg/dL), daratumumab monotherapy or in combination with bortezomib-based therapy resulted in hematological response in seven (87.5%) patients.82 The median estimated GFR at baseline was 30 mL/min, and although daratumumab resulted in kidney response in only 25% of patients, it prevented the progression of kidney disease in six out of seven patients.
Proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID): PGNMID is rare. It is specific since its involvement is kidney-limited. It is characterized by the deposition of the intact mIg; the detection rate of mIg in serum, and urine <30% or detection of PC/B-cell clones in bone marrow <10% is minimal. A clinical dilemma is common in treating PGNMID.78 Should chemotherapy be pursued in patients with advanced CKD but ineligible for KT? What should be the hematological target while treating patients with advanced CKD from PGNMID who are eligible for KT but do not have documented monoclonal Ig in serum/urine or plasma cell clones in bone marrow? What should be the timing of ASCT concerning monoclonal Ig in patients with PGNMID with advanced kidney failure? Should patients with early CKD and mild proteinuria due to PGNMID be treated with chemotherapy?
Figure 5 shows the first-line treatment of MCN, AL amyloidosis, and LCDD (adapted from Dimopoulos et al.,38 Leung et al.,54 and Kastritis et al.71), Table 5 shows common anti-plasma cell agents used in treating MM. Chimeric antigen receptor (CAR) T-cell therapy: B cell maturation antigen (BCMA)-targeted CAR T-cell therapy has shown promising results in treating relapsed/refractory multiple myeloma (RRMM).83,84 A study of 59 RRMM patients receiving BCMA-targeted CAR-T cell treatment found no significant difference in objective response rate between those with impaired kidney function (eGFR <90) and those with normal kidney function.85 Thus, it can be speculated that the utilization of CAR-T cell therapy may lead to an enhancement in kidney function in treating RRMM.
Selective inhibitors of nuclear export (SINEs)
Selinexor, a selective inhibitor of nuclear export (SINE), functions by blocking the activity of exportin 1 (XPO1), thereby inducing the accumulation and activation of tumor suppressor proteins within the cell nucleus. It exhibits inhibitory effects on nuclear factor-κB (NF-κB) and reduces the translation of oncoprotein messenger RNA.86 Selinexor holds promise as an innovative therapeutic approach for the treatment of RRMM. A subgroup analysis was conducted on the phase 3 BOSTON study, which noted a significant improvement in the overall kidney response rates among patients diagnosed with RRMM regardless of their kidney function.87
Antibody-drug conjugates (ADCs)
Antibody-drug conjugates (ADCs) are a class of therapeutic agents that consist of cytotoxic drugs bound to monoclonal antibodies designed to target specific tumor antigens. These exhibit extracellular binding through the antibody to a specific antigen, leading to the internalization of the ADCs by tumor cells.88 Upon internalization into the intracellular environment, the ADCs undergo lysosomal breakdown, leading to the subsequent liberation of the cytotoxic payload within the neoplastic cells. Belantamab mafodotin (Bela) is the first FDA-approved ADC.89 In a post hoc analysis of the DREAMM 2 study, patients with mild to moderate kidney impairment who received treatment with Bela as a single drug had comparable efficacy and safety outcomes to patients with normal kidney function.90
Unmet needs in managing multiple myeloma
There are several unmet needs in the management of MM. The MM occurs at a median age of 65 years. The presence of CKD also increases at this age, which may, independent of MM, reduce the eGFR to less than 40 mLl/min/1.73 m2. Hence, a low eGFR in a patient with a PCD is not conclusive of kidney involvement.4 We lack a non-invasive investigation for diagnosing PCD-induced kidney disease. Comorbid illnesses such as hypertension and diabetes frequently coexist in patients with MM which contribute to CKD. This mandates a kidney biopsy to identify the extent of kidney involvement in these individuals affected by MM, except in cases of Fanconi’s anemia and AL amyloidosis (fat biopsy can be used). MGRS disorders are plagued by many gaps in knowledge, which makes the management of MGRS disorders difficult. Paraprotein detectability, MDE, and PC presence in the bone marrow differ across studies published on MGRS.3,22,38,91 While the hematologic progression to overt myeloma is not high, renal progression is frequent in MGRS, eventually leading to end stage kidney diseases.91 Several small studies in MGRS noted an improved kidney outcome with clone-directed therapy.1 There is an urgent need for a concerted effort in devising standard treatment guidelines for MGRS.92 This represents an important challenge in treating the disease. The long-term survival with currently available therapies is on the rise, but still not very great considering the frequent relapses and repeated need for treatment.3
Although multiple new tools are continuously emerging for the diagnosis of multiple myeloma, such as MALDI, NGS, and peptidome technology, as described in Table 3, their practical applicability in the clinical field, especially in resource-limited settings, remains to be fully established.93-101 We have yet to determine whether the results of trials102-105 on the role of extracorporeal therapies, discussed in Table 4, have conclusively settled the matter or if further exploration is necessary.
| Mass spectrometry36,37,93 |
The key principle is the identification of M-proteins based on the unique sequence of the CDR of Ig. The clonotypic technique exhibits a high degree of analytical sensitivity, enabling the detection of M-proteins at concentrations as low as 0.001 g/L. More sensitive than conventional electrophoretic methods. It has higher sensitivity to detect minimal residual disease. |
| MALDI-TOF MS-based approach | M-proteins can be promptly identified and classified based on the distinctive mass signature of various immunoglobulin isotypes.94 By quantifying clonotypic peptides, the MS-MRD assay can be employed for determining M-protein and t-mAb quantities. The MS-MRD assay is highly sensitive in the quantification of both the M-protein and t-mAbs in real time. |
| Serum peptidome technology | The application of serum peptidome technology to identify peptides associated with kidney impairment in MM may offer a novel approach for the early evaluation and diagnosis of kidney impairment. In an analysis, the sensitivity and specificity of the test were found to be 97.14% and 94.12%, respectively.95 |
| Next-generation sequencing (NGS) or multiparameter flow cytometry (MFC)96 | The detection of malignant PC clones by NGS or MFC circumvents difficulties like interference in SPEP by m-tabs and can be performed in the peripheral blood by targeting circulating cell-free DNA or circulating plasma cells, thus also avoiding an invasive sampling procedure. This is of particular importance in monitoring patients with serologically negative diseases |
| Integration of artificial neural (ANN) networks with MS | MS coupled with ANN can provide a minimally invasive approach for the diagnosis of MM. The utilization of informative patterns in MS as inputs for ANN networks has resulted in the successful prediction of MM samples with high levels of sensitivity (100%), specificity (95%), and accuracy (98%).97 |
| Activin A | A cytokine of multifunctional properties, classified under the TGF-β superfamily. It regulates the growth and differentiation of cells in various organs.98 Urinary activin A is under research as a potential biomarker for early detection of kidney injury in MM patients as it reflects tubular injury.99 |
| Urinary exosomes100,101 | Urinary exosomes contain both surface-bound proteins and intracellular proteins that are shown in the cell’s innate orientation. Urinary exosomes from AL patients were shown to have monotypic LC oligomers adhering to the surface of exosomes from the glomerular fractions. Only monomeric LCs were detected in exosomes from patients with MM, indicating that urinary exosomes are particularly specific for AL. Urinary exosomes are being studied as a biomarker for kidney responsiveness in MGRS related diseases. |
ATN: Acute tubular necrosis; CSH: Crystal-storing histiocytosis, Glomerulonephritis, MIDD: monoclonal immunoglobulin deposition diseases; PGNMID: Proliferative glomerulonephritis with monoclonal IgG deposits; LCPT: Light chain proximal tubulopathy GN; LCCN: light chain cast nephropathy; MDE: Myeloma defining event; MGRS: Monoclonal gammopathy of renal significance; CDR: Complementarity-determining regions; Ig: Immunoglobulin; LCs: Light chains; MS: Mass spectrometry; MALDI-TOF: Matrix-assisted laser desorption ionization–time-of-flight; MRD: Minimum residual disease; t-mAbs: Therapeutic monoclonal antibodies; SPEP: serum protein electrophoresis.
| Study, author, year | Type of study | Conclusion | Highlight(s) |
|---|---|---|---|
| Zucchelli et al., 1988102 | RCT (N=29) | PLEX with chemotherapy was better in reducing Bence Jones proteinuria, kidney recovery, and 1-year survival than chemotherapy-only. | The comparator arm was PD and mortality was very high in the PD arm, limiting any conclusion on kidney recovery. |
| Johnson et al., 1990103 | RCT (N=21) | PLEX with chemotherapy reduced myeloma protein faster than chemotherapy-only | All dialysis-dependent patients who recovered were on the PLEX arm. |
| Clark et al., 2005104 | RCT (N=91) | The primary outcome, death, dialysis dependence, or eGFR <30 mL/min/1.73 m2, was similar in the PLEX and control groups. | Information on kidney biopsy diagnosis and FLC monitoring were missing. |
| Bridoux et al., 2017105 | RCT (N=98) | Hemodialysis independence was similar in the HCO-HD group and conventional (high-flux) HD group at 3 months but was better in the HCO group at 6 months. | Daily 5-hour better tolerated than daily 8-hour sessions; might be underpowered to detect a difference at 3 months. |
| Hutchison et al., 201953 | RCT (N=90) | Hemodialysis independence was similar in the HCO-HD group and conventional (high-flux) HD group at 90 days. | More infections were noted in the HCO group. |
eGFR: Estimated glomerular filtration rate; FLC: Free light chain; PLEX: Plasmapheresis; PD: Peritoneal dialysis; HD: Hemodialysis; HCO: High cut-off; RCT: Randomized control trial.
| Chemotherapy agent | Recommended dose | Kidney modification | Adverse effects |
|---|---|---|---|
| Bortezomib | 1.3 mg/m2 IV or SC on Days 1, 4, 8, and 11 | No dose adjustment is necessary | Peripheral neuropathy, myelosuppression, diarrhea |
| Carfilzomib | Cycle 1: 20 mg/m2 IV on Days 1 and 2, then 56 mg/m2 IV on Days 8, 9, 15, and 16; Cycle 2 onwards: 56 mg/m2 IV on Days 1, 2, 8, 9, 15, and 16 | No dose adjustment is necessary | Cardiovascular events, infusion reactions, myelosuppression |
| Lenalidomide | 25 mg orally once daily for 21 days of a 28-day cycle | CrCl 30 to 60 mL/min: 10 mg once daily; CrCl 15 to 30 mL/min: 15 mg once every other day; CrCl <15 mL/min or on dialysis: 5 mg once daily (after dialysis)^ | Myelosuppression, fatigue, constipation, deep vein thrombosis |
| Pomalidomide | 4 mg orally once daily for 21 days of a 28-day cycle | CrCl ≥45 mL/min: No adjustment; CrCl<45 mL/min: insufficient data; Hemodialysis: 3 mg daily (after dialysis)^ | Myelosuppression, fatigue, nausea, diarrhea |
| Cyclophosphamide | 300–400 mg/m2 IV or orally once every 28 days | CrCl <10 mL/min: 25% reduction and/or close monitoring for toxicity*, dose after hemodialysis | Myelosuppression, nausea, vomiting, hemorrhagic cystitis |
| Melphalan | 0.15 mg/kg orally once every 6 weeks | CrCl >50 mL/min: No adjustment; CrCl 10–50 mL/min: 25% reduction; CrCl <10 mL/min: 50% reduction* | Myelosuppression, gastrointestinal symptoms, increased risk of secondary malignancies |
| Doxorubicin | 9–20 mg/m2 IV once every 28 days | No adjustment; Hemodialysis: Administer after dialysis | Cardiotoxicity, myelosuppression, alopecia |
| Vincristine | 1.4 mg/m2 IV on day 1 | No dose adjustment required | Peripheral neuropathy, extravasation, constipation |
| Dexamethasone | 20–40 mg/week | No dose adjustment required | Hyperglycemia, gastritis, mood changes, adrenal suppression |
| Daratumumab |
Weeks 1 to 6: IV: 16 mg/kg once weekly for six doses; Weeks 7 to 54: IV: 16 mg/kg once every 3 weeks for 16 doses; Week 55 and beyond: IV: 16 mg/kg once every 4 weeks until disease progression |
No dose adjustments; CrCl <20 mL/min: insufficient data |
Infusion reactions, cytopenia, fatigue |
CrCl: Creatinine clearance; RCT: Randomized control trial; SC: Subcutaneous. *Aronoff GM, Bennett WM, Berns JS, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children, 5th ed, American College of Physicians, 2007; ^The United States Prescribing Information for Cyclophosphamide.
MM is a common hematologic malignancy that has a profound effect on the kidneys. Progress in the treatment of MM has led to better long-term survival of patients with kidney injury. As new anti-myeloma agents are being utilized, we are learning about novel kidney complications of those agents as well. Challenges remain in terms of diagnosis and treatment of MGRS especially the ones where the clone detection rate is low. MM is one of the most expensive cancers to treat and newer therapies, while carrying a great hope continue to be an elusive fruit to many.
Conflicts of interest
There are no conflicts of interest.
References
- Multiple myeloma current treatment algorithms. Blood Cancer J. 2020;10:94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment of multiple myeloma: ASCO and CCO joint clinical practice guideline. JCO. 2019;37:1228-63.
- [CrossRef] [PubMed] [Google Scholar]
- Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clinic Proceed. 2003;78:21-33.
- [CrossRef] [PubMed] [Google Scholar]
- Current trends of renal impairment in multiple myeloma. Kidney Dis. 2015;1:241-57.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Aetiology and management of acute kidney injury in multiple myeloma. Nephrol Dial Transplant. 2018;33:722-4.
- [CrossRef] [PubMed] [Google Scholar]
- International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538-48.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding and interpreting serum protein electrophoresis. Am Fam Physician. 2005;71:105-12.
- [PubMed] [Google Scholar]
- Does my patient with a serum monoclonal spike have multiple myeloma? Hematol Oncol Clin North Am. 2012;26:383-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical characteristics and outcomes in biclonal gammopathies. Am J Hematol. 2016;91:473-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Non-secretory myeloma: Ready for a new definition? Mediterr J Hematol Infect Dis. 2017;9:e2017053.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Unusual myelomas: A review of IgD and IgE variants. Oncol (Williston Park). 2013;27:798-803.
- [Google Scholar]
- Capillary zone electrophoresis is a very sensitive method for diagnosis and monitoring of multiple clones of multiple myeloma, other plasma cell dyscrasias and lymphoproliferative disorders. Clin Lymphoma Myeloma Leuk. 2015;15:e120-1.
- [Google Scholar]
- Laboratory assessment of multiple myeloma. In: Advances in clinical chemistry [Internet]. Elsevier; 2019. p. :1-58. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0065242318300738 [accessed 2023 Oct 16]
- [Google Scholar]
- Recognition and management of common, rare, and novel serum protein electrophoresis and immunofixation interferences. Clin Biochem. 2018;51:72-9.
- [CrossRef] [PubMed] [Google Scholar]
- Monitoring the M-protein of multiple myeloma patients treated with a combination of monoclonal antibodies: The laboratory solution to eliminate interference. Clin Chem Lab Med. 2021;59:1963-71.
- [CrossRef] [PubMed] [Google Scholar]
- A novel approach to remove interference of therapeutic monoclonal antibody with serum protein electrophoresis. Clin Biochem. 2020;75:40-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The evaluation of monoclonal gammopathy of renal significance: A consensus report of the international kidney and monoclonal gammopathy research group. Nat Rev Nephrol. 2019;15:45-59.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A new approach for rapid detection and typing of serum monoclonal components. Clin Chim Acta. 2000;302:105-24.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of fully automated and semiautomated systems for protein immunofixation electrophoresis. J Clin Lab Anal. 2017;31:e22027.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Identification of amyloidogenic light chains requires the combination of serum-free light chain assay with immunofixation of serum and urine. Clin Chem. 2009;55:499-504.
- [CrossRef] [PubMed] [Google Scholar]
- Monoclonal gammopathy detection and current technologies. In: Cancer biomarkers [Internet]. Elsevier; 2022. p. :173-201. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128243022000059 [accessed 2023 Oct 16]
- [Google Scholar]
- Serum free light chain analysis and urine immunofixation electrophoresis in patients with multiple myeloma. Clin Cancer Res. 2005;11:8706-14.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary proteins in multiple myeloma: Correlation with clinical parameters and diagnostic implications. Ann Hematol. 2003;82:487-91.
- [PubMed] [Google Scholar]
- Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: Relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437-44.
- [PubMed] [Google Scholar]
- Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361:489-91.
- [CrossRef] [PubMed] [Google Scholar]
- The tumor kinetics of multiple myeloma following autologous stem cell transplantation as assessed by measuring serum-free light chains. Leuk Lymphoma. 2006;47:21-8.
- [CrossRef] [PubMed] [Google Scholar]
- The association of serum-free light-chain levels with markers of renal function. Renal Failure. 2015;37:1057-60.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1684-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum free light chain measurement aids the diagnosis of myeloma in patients with severe renal failure. BMC Nephrol. 2008;9:11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of the serum-free light chain test in untreated patients with AL amyloidosis. Haematologica. 2008;93:459-62.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of monoclonal antibody based serum immunoglobulin free light chain immunoassays in myeloma cast nephropathy. BMC Clin Pathol. 2012;12:12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical comparison of new monoclonal antibody-based nephelometric assays for free light chain kappa and lambda to polyclonal antibody-based assays and immunofixation electrophoresis. Clin Chem Lab Med.. 2012;50:489-95.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of two serum free light chain assays for the diagnosis of primary plasma cell malignant proliferative disease. Health Sci Rep. 2019;2:e113.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparison of three different serum-free light-chain assays—implications on diagnostic and therapeutic monitoring of multiple myeloma. Blood Cancer J. 2020;10:2.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clonotypic light chain peptides identified for monitoring minimal residual disease in multiple myeloma without bone marrow aspiration. Clin Chem. 2016;62:243-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: An international myeloma working group mass spectrometry committee report. Blood Cancer J. 2021;11:24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Management of multiple myeloma-related renal impairment: Recommendations from the international myeloma working group. Lancet Oncol. 2023;24:e293-311.
- [CrossRef] [PubMed] [Google Scholar]
- International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467-73.
- [CrossRef] [PubMed] [Google Scholar]
- Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Internat. 2008;73:1282-8.
- [CrossRef] [PubMed] [Google Scholar]
- A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325-32.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic predictors of renal outcomes in light chain cast nephropathy: A multicenter retrospective study. Blood. 2020;135:1833-46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Incidence, prognostic impact and clinical outcomes of renal impairment in patients with multiple myeloma: A population-based registry. Nephrol Dial Transplant. 2021;36:482-90.
- [CrossRef] [PubMed] [Google Scholar]
- Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: A subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99:148-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Randomized trial comparing double versus triple bortezomib-based regimen in patients with multiple myeloma and acute kidney injury due to cast nephropathy. JCO. 2020;38:2647-57.
- [CrossRef] [PubMed] [Google Scholar]
- Lenalidomide and dexamethasone in patients with relapsed multiple myeloma and impaired renal function: PrE1003, a PrECOG study. Blood Cancer J. 2018;8:86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123-32.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of renal impairment in MM-003, a phase III study of pomalidomide + low-dose dexamethasone versus high-dose dexamethasone in refractory or relapsed and refractory multiple myeloma. Haematologica. 2016;101:872-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pomalidomide plus low-dose dexamethasone in patients with relapsed/refractory multiple myeloma and renal impairment: Results from a phase II trial. JCO. 2018;36:2035-43.
- [CrossRef] [PubMed] [Google Scholar]
- Daratumumab and its potential in the treatment of multiple myeloma: Overview of the preclinical and clinical development. Ther Adv Hematol. 2015;6:120-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety and efficacy of daratumumab in dialysis-dependent renal failure secondary to multiple myeloma. Haematologica. 2018;103:e277-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum free light chain levels and renal function at diagnosis in patients with multiple myeloma. BMC Nephrol. 2018;19:178.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol. 2011;22:1129-36.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Multiple myeloma with acute light chain cast nephropathy. Blood Cancer J. 2023;13:46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Online high-efficiency haemodiafiltration achieves higher serum free light chain removal than high-flux haemodialysis in multiple myeloma patients: Preliminary quantitative study. Nephrol Dial Transplant. 2011;26:3627-33.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term survival for myeloma after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2020;26:S238-9.
- [Google Scholar]
- Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: A Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement. Bone Marrow Transplant. 2019;54:353-67.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019;9:44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Autologous stem cell transplantation for multiple myeloma patients with chronic kidney disease: A safe and effective option. Bone Marrow Transplant. 2022;57:959-65.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Autologous hematopoietic cell transplantation for multiple myeloma patients with renal insufficiency: A center for international blood and marrow transplant research analysis. Bone Marrow Transplant. 2017;52:1616-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kidney transplant outcomes of patients with multiple myeloma. Kidney Int Rep. 2022;7:752-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kidney transplantation for active multiple myeloma or smoldering myeloma: A case-control study. Clin Kidney J. 2021;14:156-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kidney transplant in the era of modern therapy for multiple myeloma. Transplantation. 2018;102:1994-2001.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of relapse of multiple myeloma following kidney transplantation. Clin Kidney J. 2019;12:216-23.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Multiple myeloma with high-risk cytogenetics and its treatment approach. Int J Hematol. 2022;115:762-77.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: A meta-analysis. Bone Marrow Transplant. 2016;51:1565-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Multiple myeloma and kidney transplantation: The beginning of a new era. Clin Kidney J. 2019;12:213-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Long-term outcome of kidney transplantation in AL amyloidosis. Kidney Int. 2019;95:405-11.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of renal transplantation in patients with AL amyloidosis: An international collaboration through The International Kidney and Monoclonal Gammopathy Research Group. Blood Cancer J. 2022;12:119.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385:46-58.
- [CrossRef] [PubMed] [Google Scholar]
- Safety, tolerability, and response rates of daratumumab in relapsed AL amyloidosis: Results of a phase 2 study. Blood. 2020;135:1541-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- End-stage renal disease in systemic amyloidosis: Clinical course and outcome on dialysis. Am J Nephrol. 1990;10:283-9.
- [CrossRef] [PubMed] [Google Scholar]
- A study from the Mayo Clinic evaluated long-term outcomes of kidney transplantation in patients with immunoglobulin light chain amyloidosis. Kidney Int. 2021;99:707-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Randall-type monoclonal immunoglobulin deposition disease: Novel insights from a nationwide cohort study. Blood. 2019;133:576-87.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of light chain deposition disease: A systematic review. J Hematol. 2022;11:123-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Autologous stem cell transplant for light chain deposition disease: Incorporating bortezomib to the induction therapy. Am J Hematol. 2012;87:822-3.
- [CrossRef] [PubMed] [Google Scholar]
- How I treat monoclonal gammopathy of renal significance (MGRS) Blood. 2013;122:3583-90.
- [CrossRef] [PubMed] [Google Scholar]
- Managing light chain deposition disease. Leuk Lymphoma. 2012;53:183-4.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcome of renal transplantation in light-chain deposition disease. Am J Kidney Dis. 2004;43:147-53.
- [CrossRef] [PubMed] [Google Scholar]
- Consolidation with a short course of daratumumab in patients with AL amyloidosis or light chain deposition disease. Amyloid. 2021;28:259-66.
- [CrossRef] [PubMed] [Google Scholar]
- Daratumumab in light chain deposition disease: Rapid and profound hematologic response preserves kidney function. Blood Advances. 2020;4:1321-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front Immunol. 2023;14:1101495.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety and efficacy of chimeric antigen receptor T-cell therapy in relapsed/refractory multiple myeloma with renal impairment. Bone Marrow Transplant. 2020;55:2215-18.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-BCMA CAR-T cell therapy in relapsed or refractory multiple myeloma patients with impaired renal function. Curr Med Sci. 2021;41:474-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Selinexor and the selective inhibition of nuclear export: A new perspective on the treatment of sarcomas and other solid and non-solid tumors. Pharmaceutics. 2021;13:1522.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and tolerability of once-weekly selinexor, bortezomib, and dexamethasone in comparison with standard twice-weekly bortezomib and dexamethasone in previously treated multiple myeloma with renal impairment: Subgroup analysis from the BOSTON study. Am J Hematol. 2022;97:E83-E86.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Sig Transduct Target Ther. 2022;7:93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- FDA granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma [accessed 2023 Oct 20].
- Longer term outcomes with single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13-month follow-up from the pivotal DREAMM-2 study. Cancer. 2021;127:4198-212.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dynamic monitoring of myeloma minimal residual disease with targeted mass spectrometry. Blood Cancer J. 2023;13:30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- MALDI-TOF-MS for rapid screening analysis of M-protein in serum. Front Oncol. 2022;12:1073479.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum peptidome based multiple myeloma renal impairment biomarker screening. Blood. 2015;126:2968.
- [CrossRef] [PubMed] [Google Scholar]
- Monitoring multiple myeloma in the peripheral blood based on cell-free DNA and circulating plasma cells. Ann Hematol. 2022;101:811-24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rapid discrimination of multiple myeloma patients by artificial neural networks coupled with mass spectrometry of peripheral blood plasma. Sci Rep. 2019;9:7975.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The biology of activin: Recent advances in structure, regulation and function. J Endocrinol. 2009;202:1-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Activin a: A novel urinary biomarker of renal impairment in multiple myeloma. Biosci Rep. 2019;39:BSR20190206.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Urinary exosomes: Emerging therapy delivery tools and biomarkers for urinary system diseases. Biomed Pharmacother. 2022;150:113055.
- [CrossRef] [PubMed] [Google Scholar]
- Differences in immunoglobulin light chain species found in urinary exosomes in light chain amyloidosis (AL) PLoS ONE. 2012;7:e38061.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int. 1988;33:1175-80.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of renal failure associated with multiple myeloma. Plasmapheresis, hemodialysis, and chemotherapy. Arch Intern Med. 1990;150:863-9.
- [Google Scholar]
- Plasma exchange when myeloma presents as acute renal failure: A randomized, controlled trial. Ann Intern Med. 2005;143:777.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of high-cutoff hemodialysis vs conventional hemodialysis on hemodialysis independence among patients with myeloma cast nephropathy: A randomized clinical trial. JAMA. 2017;318:2099.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








