Translate this page into:
Revisiting Vasculopathy in Lupus Nephritis: A Renal Biopsy Evaluation Study
Address for correspondence: Dr. Megha S. Uppin, Department of Pathology, Nizam‘s Institute of Medical Sciences, Punjagutta, Hyderabad - 500 082, Telangana, India. E-mail: drmegha_harke@yahoo.co.in
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The classification of lupus nephritis (LN) on biopsy is essentially focused on morphologic changes in glomeruli. Renal vascular lesions are not addressed in detail in current classifications and are often overlooked. We aimed to determine the prevalence of vascular lesions in LN on biopsies and to compare these with biopsies not showing the vasculopathies.
Methods:
A total of 740 renal biopsies of LN were analysedfor presence of vasculopathies from January 2013 to June 2019. Of these, 527 (71.2%) biopsies showed vascular lesions (vascular group), which were further categorized into known five subtypes according to morphology and immunofluorescence (IF) findings. Remaining 213 (28.8%) biopsies constituted non-vascular group. Clinical, demographic and laboratory parameters were compared between these two groups.
Results:
The mean age was 27.95 ± 9.8 years and 27.0 ± 9.4 years in the vascular and non-vascular groups respectively with higher M:F (1:2 > 1:7) in vascular group. Majority of vasculopathies (257, 48.7%) were found in biopsies with class IV LN. Haematuria (69.8% vs. 20.1%), proteinuria (100% vs. 62%), anemia (48.3% vs. 3.60%) and hypertension (39.8% vs. 8.46%) were common in group I. Uncomplicated vascular immune deposits (426; 80.8%) were the most common vasculopathy and true vasculitis (4;0.8%) was least common. Activity and chronicity indices (7.35 ± 3 and 2.45 ± 1.5, respectively) were significantly higher in the vascular group. Activity index was highest in uncomplicated vascular immune deposits (7.45 ± 2.8) and chronicity index was highest in non-specific sclerotic vascular lesions (2.7 ± 1.6).
Conclusion:
Vascular involvement is common in LN. Uncomplicated vascular immune deposits were common vasculopathies whereas true vasculitis was least common. The morphology and IF both need to be carefully screened for the diagnosis of vasculopathies.
Keywords
Lupus nephritis
lupus vasculopathy
renal biopsy
vasculitis
Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease that has protean manifestations and follows a relapsing and remitting course. The renal complications of SLE pose risk for mortality and morbidity. Vascular changes in LN have been suggested as predictors of poor prognosis and aggressive course.[1] However, they have not been included as a part of activity and chronicity indices given by ISN/RPS classification of LN.[23] The renal vascular lesions include uncomplicated vascular immune deposits, non-inflammatory necrotizing vasculopathy (NNV), thrombotic vasculopathy/microangiopathy (TMA), true inflammatory vasculitis, and nonspecific arteriosclerosis.[4] High association of intra renal vascular lesions in SLE was first described in 1941 by Klemperer et al. in an autopsy study of patients with SLE.[5] Occurrence of true inflammatory vasculitis warrants an aggressive immunosuppressive therapy. Biopsy diagnosis of these renal vascular lesions needs a thorough examination by light, immunofluorescence (IF) and electron microscopy (EM). The vascular changes can also be attributed to complications of SLE like antiphospholipid antibody syndrome (APLA), thrombotic thrombocytopenic purpura (TTP), renal vein thrombosis and accelerated hypertension. These lesions can be easily missed in diagnostic renal biopsies owing to focal distribution of the lesions. In this study, we reviewed renal biopsies of LN cases for the presence of vasculopathies and compared vasculopathic and non-vasculopathic groups with respect to demographic, clinical and pathological parameters.
Materials and Methods
All the biopsies of LN over a period of six and half years from January 2013 to June 2019 were reviewed for the presence and types of vasculopathies. Total 740 renal biopsies were performed in the study period for the diagnosis and classification of LN in known patients of SLE. The renal involvement manifested clinically in the form of abnormal urinalysis having proteinuria or haematuria or renal failure. All 740 patients fulfilled at least four of the criteria of the American Rheumatism Association for the diagnosis of SLE.[67] The medical records of all patients were reviewed and following clinical data wererecorded at the time of biopsy: Age, sex, blood pressure, prevalence of relevant clinical manifestations with duration during the course of the disease. The following clinical features were obtained at the time of diagnosis: Anaemia, muco-cutaneous disease, arthritis, spontaneous abortions or intrauterine deaths and presence of arterial and venous thrombotic episodes.
The following laboratory data was also recorded: Haematuria, 24-hour urinary proteinuria, urinary sediments, serum albumin, serum creatinine, haemoglobin, white blood cell and platelet count, antibodies (anti-DNA, ANA, anti-Cardiolipin), C3 and C4 complement levels and presence of lupus anticoagulant.
The biopsies were re-evaluated by both light microscopy (LM) and IF and classified into different vascular lesions. For LM, the slides were reviewed with Hematoxylin and Eosin, Periodic acid-Schiff (PAS), Masson trichrome, and Silver methenamine stains. For IF microscopy, polyclonal FITC labelled (DAKO, DENMARK) antibodies against immunoglobulins (IgA, IgM, IgG), light chains (κ, λ) and complements (C1q, C3) were used. Granular staining within the vessel wall was taken as positive for vascular lesion and was graded from 0-4 according to the intensity of staining.[8] The images of the IF were captured with the help of computer software attached to the Zeiss IF microscope. These stored images were re-reviewed. The biopsies were reported by a single experienced nephropathologist (MU).
The renal biopsies were classified according to the revised ISN/RPS classification and were scored semi-quantitatively for activity and chronicity.[3] Activity scores (0–24) and chronicity scores (0–12) were calculated for all proliferative LN. Special attention was given to vascular lesions which were further categorized into the known 5 subtypes according to morphology of vascular lesions and IF findings. The descriptions given by Ding et al.[4] were appropriately followed for classification of vasculopathies.
Statistical analysis
Statistical analysis was done on SPSS software version 20. Results were given as range, mean and percentage positivity. Unpaired t test was used for two independent groups and Chi-square test was used for test of association between categorical variables. Means of activity and chronicity indices were compared for statistical significance using one-way analysis of variance (ANOVA) and Tukey's multiple comparison tests. A value of P < 0.05 was considered statistically significant.
Results
The revised ISN/RPS classification of the LN in the 740 biopsies is provided in Table 1. Proliferative LN including Class III and class IV were the dominating numbers together accounting for 71.3% (528) biopsies. Out of 740 biopsies, 527 (71.2%) were found to have vascular involvement and were included under “vascular group”, whereas the remaining 213 (28.8%) formed the “non-vascular group.” The types of vasculopathies included uncomplicated vascular immune deposits (426; 80.8%), NNV (34; 6.4%), TMA (16; 3.0%), true inflammatory vasculitis (4; 0.8%) and nonspecific sclerotic vascular lesions (47; 8.9%) as shown in Figure 1 and the IF staining of the vascular immune deposits has been depicted in Figure 2. The cases of NNV and 34 cases of nonspecific arteriosclerosis also showed vascular immune deposits. The comparison of activity index (AI) and chronicity index (CI) between the two groups and different types of vasculopathies is provided in Tables 2 and 3 respectively. Proliferative LN, including class III and IV, was dominant in both the groups. AI and CI were also significantly higher and this difference was statistically significant. All types of vasculopathies were common in proliferative LN (Class III and IV). AI was highest in uncomplicated vascular immune deposits and CI was highest in nonspecific sclerotic vascular lesions.
| Morphologic form | No. of biopsies N (n %) |
|---|---|
| Class I - Normal glomeruli or mild mesangial GN | 14 (1.9%) |
| Class II - Mesangial GN | 16 (2.2%) |
| Class III - Focal proliferative GN | 181 (24.5%) |
| Class IV - Diffuse proliferative GN | 332 (44.9%) |
| Class V - Membranous GN | 118 (15.9%) |
| Class VI - Sclerosing GN | 21 (2.8%) |
| Class II + Class V | 6 (0.8%) |
| Class III + Class IV | 15 (2.0%) |
| Class III + Class V | 20 (2.7%) |
| Class IV + Class V | 17 (2.3%) |
| Total | 740 (100%) |
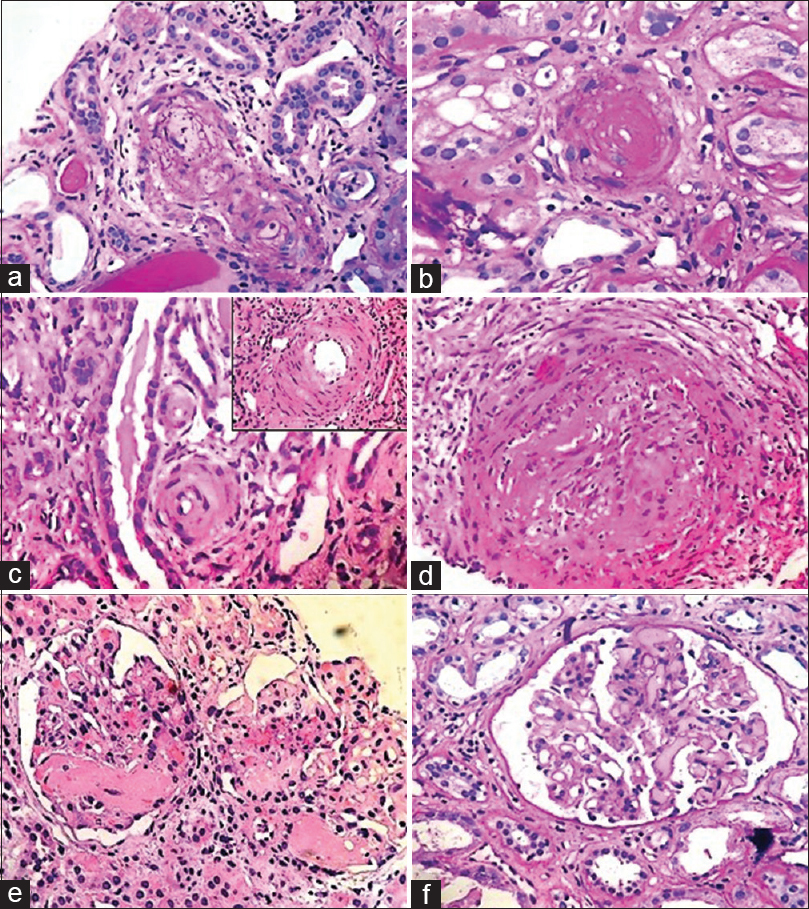
- All the types of lupus vasculopathy including (a) PAS positive Immune vascular casts in an arteriole (b) Non inflammatory necrotizing vasculopathy showing occlusion of lumen by nuclear debris and eosinophilic smudgy material in absence of inflammation (c) Non-specific sclerosis in two small arterioles (d) Vasculitis intense neutrophilic inflammatory infiltrate in vessel wall. (e) TMA affecting two glomeruli with intraluminal fibrin thrombi seen in glomerular capillaries. (f) TMA affecting a glomerulus is seen with multiple PAS positive intraluminal thrombi
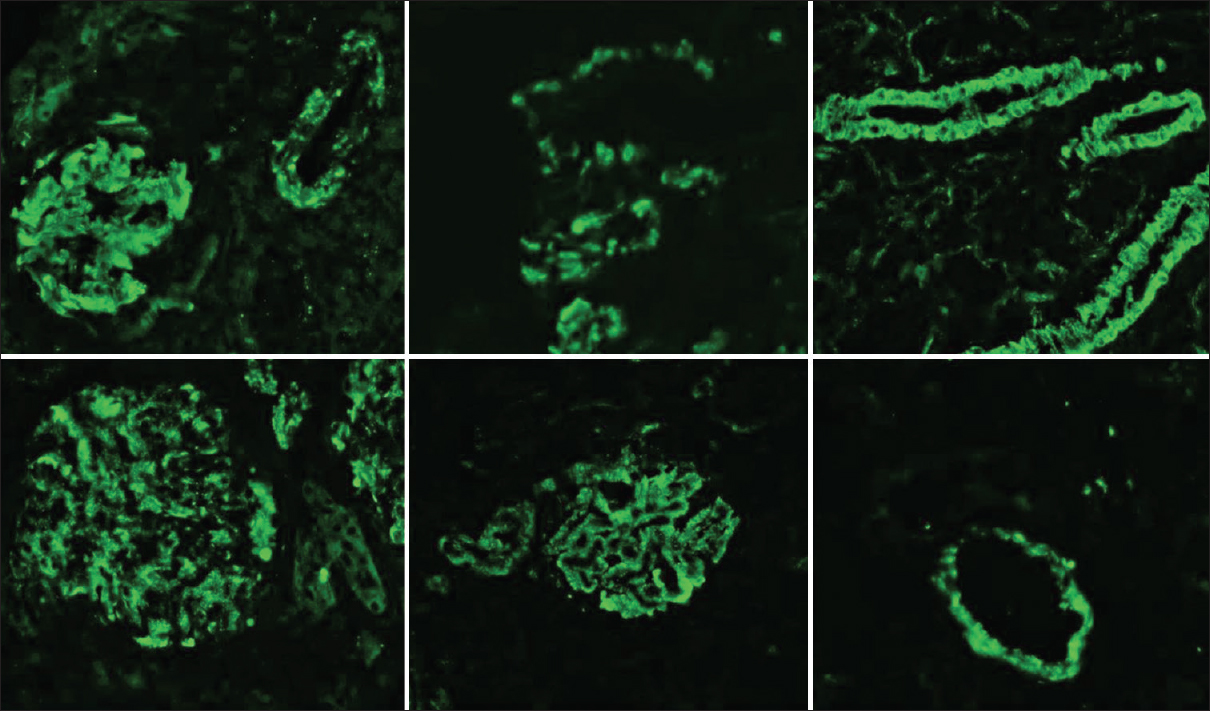
- The direct IF staining showing granular staining within the vessel walls for IgG, C3 and C1q
| Vascular group n (%) | Non-vascular group n (%) | P | |
|---|---|---|---|
| No. of patients | 527 (71.2%) | 213 (28.8%) | |
| Activity index (AI) | 7.35±3 (1-19) | 6.34±3.6 (0-15) | 0.000148 |
| Chronicity index (CI) | 2.45±1.5 (0-7) | 0.56±1.4 (0-6) | <0.00001 |
Values=Mean±SD (Range)
| Uncomplicated vascular immune casts n (%) | Non -inflammatory necrotizing vasculopathy n (%) | TMA n (%) | Vasculitis n (%) | Non-specific sclerotic vascular lesions N (n%) | P | |
|---|---|---|---|---|---|---|
| No. of patients | 426 (80.8%) | 34 (6.4%) | 16 (3.0%) | 4 (0.8%) | 47 (8.9%) | |
| AI | 7.45±2.8 (2-19) | 6.7±2.4 (0-15) | 5.5±1.9 (2-10) | 5.75±2.1 (1-14) | 5.9±2.0 (2-10) | 0.00001 |
| CI | 2.2±1.2 (0-8) | 2.4±0.9 (0-5) | 0.85±0.5 (0-2) | 0.7±0.4 (0-2) | 2.7±1.6 (0-6) | <0.00001 |
Values=Mean±SD (Range)
Using one way ANOVA, it was found that there was significant difference in the means of five groups of lupus vasculopathy for both AI and CI. Tukey's multiple comparison test showed significant differences in the means of AI between uncomplicated vascular immune deposits and nonspecific sclerotic lesions. Also, mean of CI showed statistically significant difference between uncomplicated vascular immune deposits and TMA, NNV and TMA. Rest of the vascular groups did not show any significant difference between the means of AI and CI. Table 4 compares the clinical, demographic and laboratory parameters between the two groups. There was no difference in the mean age between two groups; however, the M: F ratio was significantly higher for the vascular group. Amongst the laboratory parameters, proteinuria, hematuria, anemia and hypertension were more prevalent in the vascular group and the difference was statistically significant. The patients in vascular group had significantly low levels of hemoglobin (9.5 ± 1.9 vs. 10.4 ± 1.7 gm/dl), higher levels of serum creatinine (4.01 ± 3.3 vs. 2.35 ± 2.3mg/dl) and higher positivity for anticardiolipin antibodies (21.4% vs. 2.3%) compared to patients in non-vascular group.The serologic markers, like ANA, ds-DNA did not show any difference so as hypocomplementemia.
| Lab data | Vascular group of lesions n (%) | Non vascular group of lesions n (%) | P |
|---|---|---|---|
| No. of patients | 527 | 213 | |
| Age Mean±SD (Range) | 27.95±9.8 (5-58) | 27.0±9.4 (10-75) | NS |
| M:F | 1:2 | 1:7 | 0.0016 |
| Hemoglobin (gm/dl) | 9.5±1.9 (5.8-11.6) | 10.4±1.7 (5.6-12.6) | 0.0072 |
| Mean±SD (Range) | |||
| Serum creatinine (mg/dl) | 4.01±3.3 (0.19-13.7) | 2.35±2.3 (0.4-9.6) | 0.0012 |
| Mean±SD (Range) | |||
| Proteinuria | 527 (100%) | 132 (62%) | <0.00001 |
| Proteinuria (mg/dl) | NS | ||
| Mean±SD (Range) | 2279±1291 (300-10900) | 1981±1876 (100-9000) | |
| Nephrotic range proteinuria | 21 (4.0%) | 8 (3.8%) | NS |
| Hematuria | 368 (69.8%) | 43 (20.1%) | <0.00001 |
| Anemia | 255 (48.3%) | 8 (3.80%) | <0.00001 |
| Hypertension | 210 (39.8%) | 18 (8.46%) | <0.00001 |
| Arthritis | 140 (26.5%) | 41 (19.43%) | NS |
| ANA +ve | 402 (76.2%) | 127 (59.6%) | NS |
| DsDNA +ve | 290 (55.0%) | 90 (42.16%) | NS |
| Anticardiolipin +ve | 113 (21.4%) | 5 (2.3%) | <0.00001 |
| C3 (mg/ml) | 89.6±39.6 (8-142) | 56.1±42.3 (11-145) | NS |
| Mean±SD (Range) | |||
| C4 (mg/ml) | 19.7±10.7 (1-39) | 12.1±12.3 (1-41) | NS |
| Mean±SD (Range) | |||
| Lupus anticoagulant +ve | 5 (0.9%) | 1 (0.5%) | NS |
Table 5 compares the clinical, demographic and laboratory parameters among vascular lesions. All the patients presented with proteinuria. Patients with NNV showed highest number of cases with anemia (94.1%) and low C4 levels (mean 12 ± 8 mg/dl). Patients with TMA showed anticardiolipin and lupus anticoagulant positivity accounting for 42.8% and 31.2%, respectively. All cases with true vasculitis showed hematuria, hypertension, ANA and anti-ds-DNA antibodies positivity. Patients with non-specific sclerotic vascular lesions had proteinuria with mean of 2392 ± 1633 mg/day, lowest hemoglobin levels (7.4 ± 1.6 gm/dl), highest serum creatinine levels (mean: 6.3 ± 4 mg/dl) and low C3 levels (mean: 65 ± 42.3mg/dl).
| Clinical data | Uncomplicated vascular immune casts n (%) | Non -inflammatory necrotizing vasculopathy n (%) | TMA n (%) | Vasculitis n (%) | Non specific sclerotic vascular lesions n (%) |
|---|---|---|---|---|---|
| No.of patients | 426 (80.8%) | 34 (6.4%) | 16 (3.0%) | 4 (0.8%) | 47 (8.9%) |
| Age (years) | 27.8±9.3 (14-35) | 30±12.7 (17-50) | 29±14 (18-56) | 27.7±9 (17-37) | 28±7.2 (16-40) |
| Mean±SD (range) | |||||
| M: F | 1:4 | 2:3 | 2:5 | 1:3 | 1:1 |
| Hb | 10.54±1.4 (8.3-11.6) | 8.1±1.7 (5.8-10.2) | 9.4±2.4 (6.2-11.6) | 10.3±1.6 (7.8-11.3) | 7.4±1.6 (7.4-11.5) |
| Mean±SD (Range) | |||||
| Serum Cr. (mg/dl) | 1.32±0.7 (0.8-2.6) | 3.88±2.9 (0.8-7.5) | 3.7±2.2 (0.7-6.4) | 2.45±2.1 (0.8-2.3) | 6.3±491.7-13.7) |
| Mean±SD (Range) | |||||
| Proteinuria | 1504±1120 (600-3000) | 1464±1375 (300-3500) | 1703±947 (600-2900) | 1163±785 (500-2300) | 2392±1633 (800-6000) |
| Mean±SD (Range) | |||||
| Nephrotic range proteinuria | 0 (0%) | 7 (20.5%) | 0 (0%) | 0 (0%) | 14 (30%) |
| Hematuria | 301 (70.6%) | 23 (67.6%) | 11 (71.4%) | 4 (100%) | 29 (61.7%) |
| Anemia | 183 (42.9%) | 32 (94.1%) | 7 (42.8%) | 1 (25%) | 32 (68.0%) |
| Hypertension | 170 (39.9%) | 14 (41.1%) | 5 (31.25%) | 4 (100%) | 17 (36.1%) |
| ANA +ve | 351 (82.3%) | 21 (61.7%) | 7 (42.8%) | 4 (100%) | |
| DsDNA +ve | 255 (60%) | 12 (35.2%) | 4 (25%) | 4 (100%) | 19 (40.4%) |
| Anticardiolipin +ve | 96 (22.5%) | 0 (0%) | 7 (42.8%) | 0 (0%) | 10 (21.2%) |
| C3 (mg/ml) | 74±37 (18-102) | 70.2±37.5 (32-124) | 101.8±35.5 (36-142) | 86±45 (24-132) | 65±42.3 (25-130) |
| Mean±SD (Range) | |||||
| C4(mg/ml) | 17.2±13 (3-32) | 12±8 (2-22) | 23±7 (8-32) | 19.5±13 (2-34) | 19±12 (1-39) |
| Mean±SD (Range) | |||||
| Lupus anti coagulant +ve | 0 (0%) | 0 (0%) | 5 (31.2%) | 0 (0%) | 0 (0%) |
Discussion
Renal vascular lesions in patients with SLE have long been claimed to be associated with advanced disease and poor prognosis. However, these vascular lesions were not included in the scoring of activity or chronicity indices, which are routinely used in predicting disease progression.[9] The proposal for inclusion of renal vascular lesions in the summary diagnosis of LN was put forth by ISN/RPN in 2003.[2] The 2018 update has revised several terminologies and definitions bringing more clarity to the classification. The definition for vasculopathy is proposed as luminal narrowing of arterioles or terminal interlobular arteries by intramural immune deposits, typically admixed with fibrinoid changes, without inflammation of the vessel wall.
The studies dedicated to the lupus vasculopathies are few and most are old. The notable recent single centre study is by Wu et al.[8] who studied 279 biopsies of vasculopathies and found TMA to have worst outcome. They also proposed new activity and chronicity indices incorporating the vascular lesions, which they claimed to be significantly better predictors of renal outcome.
Comparison of the basic demographic features showed M: F ratio to be significantly higher in the vascular group (1:2 vs. 1:7) compatible with Wu et al. study.[8] The median age was 27.95 years in vascular group compared to study by Wu et al. (32.9 years).[8] The prevalence of vasculopathies (71.2%) in our study is very much comparable to that by Wu et al. who reported vascular lesions in about 81.8% of their biopsies of LN.[8] Similarly, a University lupus registry data at Toronto has reported existence of lupus vascular lesions in 75.2% of their patients.[10] The incidence of vascular lesions in the older studies is variable and perhaps cannot be reliably compared to our study.[11112]
It is also important to understand the pathogenesis of vasculopathies, which has bearing on their total incidence as well as difference in the type of vasculopathy. Ding et al.[4] in their recent review have proposed immune dysregulation as a possible initial event. Formation of autoantibodies and immune complexes results in activation of endothelial cells and trigger the complement cascade with release of cytokines.[13] This also perhaps is the reason why uncomplicated immune deposits are the most common type of vasculopathy. These accounted for 80.8% of total vascular lesions in our study and also was the dominant vasculopathy (74.2%) reported by Wu et al.[8] They also reported a poor renal outcome associated with immune deposits than with other types of vasculopathies except TMA. This was contradictory to other older studies which regarded vascular immune deposits as a begin entity which did not warrant aggressive immunosuppression.[1415] Barber et al. however reported immune complex deposits in only 6.2% of their vasculopathies.[10]
NNV accounted for 6.4% of vascular lesions in our study whereas Wu et al. reported these in 3.8% biopsies. Similar to their observation, we found that most of these lesions had immune complex deposits on IF. This consolidates the observation that NNV is a transition from vascular immune complex deposits to other severe vascular lesions, such as TMA.[811] Chu et al.[16] in their study of 142 biopsies of LN, identified NNV in 9 cases. The patients with NNV had higher proportions of noninfection leukocyturia and leukocytopenia, significantly higher serum creatinine, lower hemoglobin and serum C3 levels. These findings are comparable to our patients of NNV.
TMA is another vasculopathy which needs special attention, and whose diagnosis must not be missed owing to its poor prognosis. Presence of APLA and complement activation is responsible for TMA. Glomerular C4d deposits have been shown to be the marker of activation of classical complement pathway.[1718] We identified TMA in relatively less biopsies (3.0%) as compared to that by Wu et al.[8] who reported TMA in 17.6% and Barber et al.[10] in 8.1% biopsies. Anticardiolipin antibodies and lupus anticoagulant positivity were seen in 42.8% and 31.2% of our patients of TMA; however, we did not do the glomerular C4d studies to support the theory of complement activation. The mean serum complement levels were normal in these patients.
True vasculitis was the least frequent lesion in comparison to other studies by Wu et al. (0.6%) and Descombes et al.[812] Barber et al. did not report any case of true vasculitis.[10] Concomitant anti-neutrophil cytoplasmic antibody (ANCA) positivity has been reported in some patients of lupus showing true vasculitis.[19] However we did not identify any such association.
Atherosclerosis accounted for 8.9% of the vasculopathies whereas others have reported higher incidence of this particular vascular lesion. Wu et al.[8] identified it in 24% where as Barber et al. reported it in almost 57.8% of their biopsies. The pathogenesis of atherosclerosis is multifactorial and is attributed to chronic inflammation, antiendothelial antibodies, antibodies to HDl and also cytokine mediated endothelial damage.[2021] Highest CI was seen in atherosclerosis in comparison to other vascular lesions. This supports the association of chronicity, and longer disease duration with atherosclerosis. Whether these sclerotic vascular lesions are the cause or a consequence of chronicity is not known.
All the vascular lesions were seen to occur more commonly in proliferative LN as shown by other studies as well. There was no correlation between the class and type of vascular lesion. Class IV was the commonest LN (48.0%) compatible with studies by Wu et al. (55.1%), Barber et al. and Descombes et al.[81012] Similarly the activity and chronicity indices also were higher in the vascular group than non-vascular group compatible with studies by Wu et al. and Barber et al.[810] The majority of patients with vasculopathies showed proteinuria (100% vs. 62%), hematuria (69.8% vs. 20.1%), anemia (48.3% vs. 3.6%) and hypertension (39.8% vs. 8.46%) when compared to non-vascular group of patients. A significant higher positivity for anti-cardiolipin antibodies (21.4%vs. 2.1%) was also seen in the vascular group. This data is comparable to that of Wu et al (65.4%).[8]
The IF pattern between the vascular and non-vascular groups did not reveal any specific associations except for higher cases in the vascular group showing deposition of C1q (77.5%), IgG (74.5%) followed by C3c (70.6%) compatible with the study by Wu et al., which showed deposition of C1q (87.4%), IgG (44.3%) followed by C3c (42.7%) in the vascular group of patients.[8] However, no relevant conclusions could be drawn from these findings.
We could not draw any prognostic associations with different types of vascular lesions due to inadequate treatment and follow up data. This remains the major limitation of our study.
Conclusion
Vasculopathies are common in patients with LN. Patients in vascular group showed higher male to female ratio and had low hemoglobin and high serum creatinine levels. These patients also had higher proteinuria, hematuria, anemia, and hypertension, more proliferative LN with higher activity and chronicity index as compared to non-vascular group. Uncomplicated vascular immune deposits were the most common whereas true vasculitis was the least common. A thorough LM and IF evaluation is the key to diagnose vasculopathies in LN.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Renal vascular lesions as a marker of poor prognosis in patients with lupus nephritis. GruppoItaliano per lo studio dellanefritelupica (GISNEL) Am J Kidney Dis. 1991;18:240-8.
- [Google Scholar]
- The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am SocNephrol. 2004;15:521-30.
- [Google Scholar]
- Revision of the International society of nephrology/renal pathology society classification for lupus nephritis: Clarification of definitions, and modified National institutes of health activity and chronicity indices. Kidney Int. 2018;93:789-96.
- [Google Scholar]
- Renal involvement in systemic lupus erythematosus (SLE): A study of 56 patients emphasizing histologic classification. Medicine. 1978;57:371-408.
- [Google Scholar]
- Inclusion of renal vascular lesions in the 2003 ISN/RPS system for classifying lupus nephritis improves renal outcome predictions. Kidney Int. 2013;83:715-23.
- [Google Scholar]
- Diffuse proliferative lupus nephritis: Identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689-95.
- [Google Scholar]
- Evaluation of clinical outcomes and renal vascular pathology among patients with lupus. Clin J Am SocNephrol. 2012;7:757-64.
- [Google Scholar]
- Renal vascular complications of systemic lupus erythematosus. J Am SocNephrol. 1994;4:1499-515.
- [Google Scholar]
- Endothelial alterations in systemic lupus erythematosus and rheumatoid arthritis: Potential effect of monocyte interaction. Mediators Inflamm 2017 2017:9680729.
- [Google Scholar]
- Course of renal pathology in patients with systemic lupus erythematosus. Am J Med. 1984;77:612-20.
- [Google Scholar]
- Long-term follow-up of patients with lupus nephritis. A study based on the classification of the World Health Organization. Am J Med. 1987;83:877-85.
- [Google Scholar]
- Noninflammatory necrotizing vasculopathy in lupus nephritis: A single-center experience. Lupus. 2014;23:20-30.
- [Google Scholar]
- Potential for glomerular C4d as an indicator of thrombotic microangiopathy in lupus nephritis. Arthritis Rheum. 2008;58:2460-9.
- [Google Scholar]
- Nationwide population-based epidemiologic study of systemic lupus erythematosus in Taiwan. Lupus. 2010;19:1250-5.
- [Google Scholar]
- Anti-neutrophil cytoplasmic antibodies in new-onset systemic lupus erythematosus and lupus nephritis. Inflammation. 2008;31:260-5.
- [Google Scholar]
- Vasculopathy and vasculitis in systemic lupus erythematosus. Pol Arch Med Wewn. 2008;118:57-63.
- [Google Scholar]
- Antiendothelial cell antibodies in systemic lupus erythematosus. Autoimmun Rev. 2002;1:365-72.
- [Google Scholar]







