Translate this page into:
Rhabdomyolysis-associated Acute Kidney Injury
Address for correspondence: Dr. S Sujit, Department of Nephrology, Government Stanley Medical College and Hospital, Chennai - 1, Tamil Nadu, India. E-mail: nephrosuji@yahoo.in
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Acute kidney injury represents one of the most severe complications of rhabdomyolysis.
Methods:
We performed a prospective observational study to analyze the etiology, clinical manifestations, laboratory profile, and outcome in patients with biopsy-proven pigment-induced nephropathy between January 2017 and September 2019. History, clinical examination findings, laboratory investigations, and outcomes were recorded.
Results:
A total of 26 patients were included. Mean age was 34.81 ± 11.89 years. Mean peak serum creatinine was 6.79 ± 4.07 mg/dL. Median values of Creatine phosphokinase (CPK) and Lactate dehydrogenase (LDH) were 12500 U/L (3187, 17167.50) and 447 U/L (354.50, 908.75), respectively. Of the patients presenting with rhabdomyolysis, 12 patients (46%) had traumatic causes and 14 patients (54%) had nontraumatic causes. Nontraumatic etiology of rhabdomyolysis included seizures (1), wasp sting (1), paraphenylenediamine ingestion (2), rat killer ingestion (2), leptospirosis (2), dehydration (3), acute limb ischemia (1), Gloriosa superba ingestion (1), and prolonged immobilization (1). On renal biopsy, 16 patients had myoglobin cast nephropathy and one had immunoglobulin A deposits in addition to pigment nephropathy. Twenty (76.9%) were initiated on hemodialysis, and two patients (7.6%) were treated with peritoneal dialysis and four patients (15.5%) were treated with forced alkaline diuresis. A total of four patients died (15.4%) due to sepsis/disseminated intravascular coagulation and respiratory failure. At the mean follow-up of 6 months, two patients (7.7%) progressed to chronic kidney disease (CKD).
Conclusions:
Rhabdomyolysis-associated acute kidney injury is an important cause of renal failure requiring renal replacement therapy. In our study, it was more common in males. Traumatic and nontraumatic causes played an equal causative role. Most of the patients recovered from AKI. Forced alkaline diuresis was found useful in nontraumatic rhabdomyolysis AKI.
Keywords
Acute kidney injury
dialysis
forced alkaline diuresis
rhabdomyolysis
Introduction
Rhabdomyolysis is a clinical syndrome that results from direct or indirect injury-causing skeletal muscle disruption and leakage of potentially toxic cellular contents into the systemic circulation. Rhabdomyolysis-induced pigment nephropathy is common, accounting for about 7%–10% of all cases of acute kidney injury (AKI).[1] The causes of rhabdomyolysis-induced AKI may differ in different parts of the world. Thus, we intend to study the etiology, clinical manifestation, laboratory profile, and outcome in patients with traumatic and nontraumatic causes of rhabdomyolysis-associated nephropathy.
Methods
We performed a prospective observational study in patients presenting with rhabdomyolysis-associated AKI between January 2017 and September 2019. The study included patients who were 18 years of age and above, and were diagnosed with rhabdomyolysis associated AKI with a CPK level more than 3 times the upper normal limit (170 U/L in males and 135 U/L in females).
AKI was defined as per Kidney Disease Improving Global Outcomes 2012 guidelines, as an increase in serum creatinine by ≥0.3 mg/dL within 48 h or increase in serum creatinine to 1.5 times from baseline that is known or presumed to have occurred within the prior 7 days, or urine volume <0.5 mL/kg/h for 6 h. Complete recovery of kidney function was defined as a decrease in the serum creatinine level to within a normal range. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 at 3 months after the onset of AKI. The presence of rhabdomyolysis was diagnosed by clinical history and serum CPK >510 U/L in males, 405 U/L in females (three times the normal). Those patients with underlying known renal disease or if more than 7 days had elapsed since presentation were excluded. Renal biopsy was done in patients who had persistent oliguria for >3 days and renal failure for >3 days despite supportive treatment. Immunostaining for myoglobin was done in all biopsied samples.
History of recent trauma, exertion, seizures, infections, and intake of alcohol/medications; demographic data; and clinical findings were noted. Laboratory investigations included urine analysis, urine myoglobin, blood urea, serum creatinine, sodium, potassium, calcium, phosphorus, serum creatine phosphokinase (CPK), and lactate dehydrogenase (LDH). In addition, treatment details and outcomes were recorded. All the renal biopsies were studied under light microscopy with various stains, including hematoxylin and eosin, Periodic acid–Schiff, trichrome, and Periodic Schiff–methenamine. In the presence of intratubular pigment casts with a globular/ropy appearance, myoglobin immunohistochemistry (IHC) was done whenever possible. The etiology of rhabdomyolysis was ascertained by history, clinical, and laboratory findings.
All the patients received supportive treatment and forced alkaline diuresis was given when presented without volume overload or oliguria. Indications of dialysis were oligoanuria, refractory hyperkalemia (>5.5 mEq/L), metabolic acidosis, and acute pulmonary edema, with acute peritoneal dialysis in hemodynamically unstable patients and hemodialysis in hemodynamically stable patients.
Data were expressed as mean (± standard deviation), median (range), numbers, or percentages. Statistical analysis was done by independent T test for continuous variables following normal distribution, Chi-square test for categorical variables, and Mann–Whitney U test for nonparametric data. All calculations were performed using the SPSS software package (v15.0, SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
A total of 654 patients were documented to have AKI during the study period, out of which 8.8% (57 patients) had laboratory evidence of rhabdomyolysis. After excluding patients with underlying kidney disease and those who were lost to follow-up for more than 3 months, a total of 26 patients (3.9%) were included in the study. The demographic data are presented in Table 1. The study population had a mean age of 34.81 ± 11.89 years. Among this study population, 12 patients (46%) had traumatic causes and 14 patients (54%) had nontraumatic causes, such as wasp sting, Gloriosa superba ingestion, leptospirosis, and rat killer paste ingestion [Table 2]. Of the 12 patients who presented with traumatic cause, three patients (25%) had Rh-AKI following assault. There was only one female in the entire study group who presented with rhabdomyolysis due to paraphenylenediamine ingestion. Twenty patients (78%) presented with oliguria. The mean peak serum creatinine was 6.79 ± 4.07 mg/dL. Mean serum calcium, serum phosphorus, serum uric acid, and serum potassium was 8.82 ± 0.92 mg/dL, 4.26 ± 0.73 mg/dL, 5.22 ± 1.17 mg/dL, and 5.32 ± 0.96 mEq/L, respectively. Renal biopsy was done in 16 patients (61.5%) who had persisting renal failure or oliguria for more than 3 days to rule out other causes of AKI [Figures 1 and 2]. All renal biopsies revealed acute tubular injury in dilated tubules, swollen tubular epithelial cells with cytoplasmic vacuoles, sloughed off epithelial cells forming granular debris, edematous interstitium, and pigment casts that stained positive for myoglobin in the tubules. None of the patients had significant glomerulosclerosis, interstitial fibrosis, or tubular atrophy. Of the 16 patients who underwent biopsy, one patient with rat killer ingestion was incidentally found to have IgA nephropathy.

- Granular casts with tubular epithelial injury (hematoxylin and eosin; original magnification, X 200)
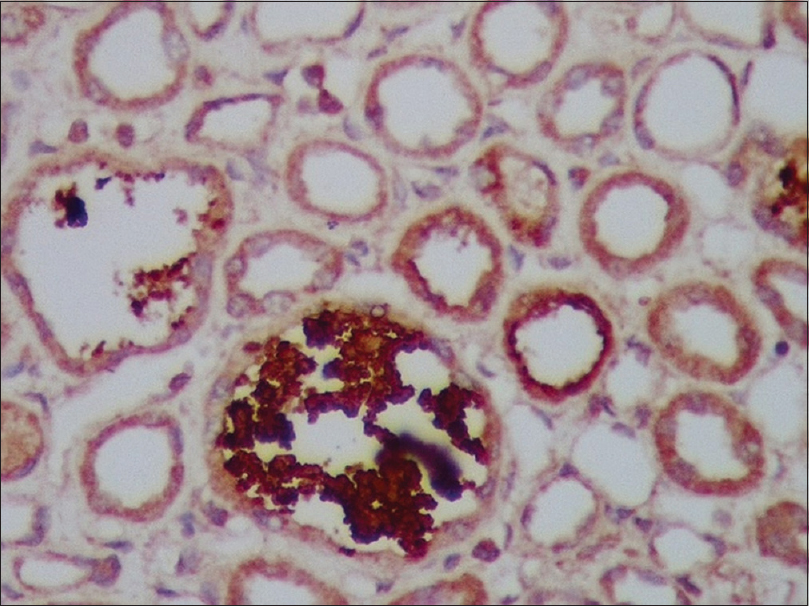
- Casts are strongly positive with anti-myoglobin antibody (immunohistochemistry staining, original magnification, x 200)
| Parameter | Study population n=26 | Trauma cases n=12 (46.2%) | Nontraumatic cases n=14 (53.8%) | P | |||
|---|---|---|---|---|---|---|---|
| Mean±SD | n (%) | Mean±SD | n (%) | Mean±SD | n (%) | ||
| Age | 34.81±11.89 | 33.83±12.80 | 35.64±11.46 | 0.7 | |||
| Gender | |||||||
| Male | 25 (96.15) | 12 | 13 | 0.3 | |||
| Female | 1 (3.8) | 0 | 1 | ||||
| Peak serum creatinine (mg/dL) | 6.79±4.07 | 7.63±4.14 | 6.07±4.01 | 0.3 | |||
| Serum uric acid (mg/dL) | 5.22±1.17 | 5.9±0.98 | 4.62±1.0 | 0.03 | |||
| Serum calcium (mg/dL) | 8.82±0.92 | 9.06±1.00 | 8.62±0.83 | 0.2 | |||
| Serum phosphorus (mg/dL) | 4.26±0.73 | 4.21±0.68 | 4.30±0.78 | 0.8 | |||
| Serum potassium (mEq/L) | 5.32±0.96 | 5.5±0.83 | 5.12±1.04 | 0.3 | |||
| Serum CPK (U/L) | 12500 (3187,17167.50) | 10382 (8710) | 13075 (43158) | 0.5 | |||
| Serum LDH (U/L) | 447 (354.50,908.75) | 660.50 (628) | 410.5 (349) | 0.17 | |||
| Biopsy done | 16 (61.5) | 7 (53.8) | 9 (64.3) | 0.7 | |||
| Peritoneal dialysis | 2 (7.7) | 1 (16.7) | 1 (7.7) | 0.7 | |||
| Patients who underwent forced alkaline diuresis | 4 (15.4) | 0 | 4 (28.6) | 0.04 | |||
| Patients who underwent hemodialysis | 20 (80.8) | 11 (83.3) | 9 (71.4) | 0.3 | |||
| Patients who recovered from AKI | 18 (69.2) | 8 (66.6) | 10 (71.4) | 1.0 | |||
| Patients who died | 4 (15.4) | 1 (8.3) | 3 (21.4) | 0.6 | |||
| Patients who progressed to Chronic kidney disease | 2 (7.7) | 1 (8.3) | 1 (7.1) | 1.0 | |||
Abbreviations: CPK, Creatinine phosphokinase, LDH, Lactate dehydrogenase
| Trauma (n=12) | Non-trauma (n=14) |
|---|---|
| Assault (3) | Paraphenylene diamine ingestion (2) |
| Fall injury (8) | Rat killer (2) |
| CPR (1) | Prolonged immobilization (1) |
| Acute limb ischemia (1) | |
| Gloriosa superba ingestion (1) | |
| Wasp sting (1) | |
| Leptospirosis (2) | |
| Dehydration (2) | |
| Seizures (1) |
On statistical analysis [Table 1] by independent T test for continuous variables following normal distribution, Chi-square test for categorical variables, and Mann–Whitney U test for nonparametric data, there was no difference between patients with traumatic and nontraumatic rhabdomyolysis, except for mean uric acid levels, which were found to be significantly higher in the traumatic group (5.9 ± 0.98 vs. 4.62 ± 1.0; P = 0.03). The utility of forced alkaline diuresis was seen in four patients, all of them belonging to the nontraumatic group (P = 0.04).
Of the 26 patients, 20 (76.9%) were initiated on hemodialysis, and two patients (7.6%) were treated with peritoneal dialysis. Four patients (15.5%) who were treated with forced alkaline diuresis belonged to the nontraumatic group and had recovered from AKI. A total of 18 patients (69.2%) had recovered from AKI, whereas four patients died (15.4%) due to sepsis/disseminated intravascular coagulation and respiratory failure.
During the study period, two patients (7.7%) had progressed to chronic kidney disease, with one patient being hemodialysis-dependent and another patient’s serum creatinine did not reach normal, though he became dialysis independent. After considering loss to follow-up (n = 2), we found no statistical difference in progression to CKD between the traumatic and nontraumatic groups [1 (10%) vs. 1 (7.1%)), P = 1.0].
Discussion
Rhabdomyolysis refers to the dissolution of striped muscle resulting in the release of muscular cell constituents into the circulation.[2] As a consequence of the muscle damage, the levels of serum myoglobin, serum creatine phosphokinase (CPK), and lactate dehydrogenase (LDH) rise. Rhabdomyolysis has been associated with AKI because myoglobin, a 17.8-kDa protein, is nephrotoxic. Various studies have quoted that approximately 10%–50% of patients with rhabdomyolysis develop AKI, and it contributes to 5%–25% of all cases of AKI.[3,4] In our study, 3.9% of patients (26) had rhabdomyolysis-associated AKI, similar to a study done by Sakthirajan et al.[5]
Myoglobin can mediate renal vasoconstriction, proximal tubular cytotoxicity, and can precipitate in the tubules, causing obstruction.[6] In an acidic environment, myoglobin that is concentrated in the renal tubules forms precipitates with the Tamm–Horsfall protein, a process favored by acidic urine. It appears in the urine only when the renal threshold of 0.5–1.5 mg/dL of myoglobin is exceeded. Tubular obstruction usually occurs at the distal tubules, and myoglobin can cause direct tubule toxicity in the proximal tubules. Though serum myoglobin levels are the earliest to rise following muscle injury, it has a rapid and unpredictable metabolism. The measurement of serum myoglobin has a low sensitivity for the diagnosis of rhabdomyolysis.[7] Thus, serum CPK is the most sensitive enzyme marker of muscle injury. There is no defined threshold value of serum CPK above which the risk of AKI is markedly increased, and values >5000 U/L have been reported to increase the risk of AKI. Similar to the studies by Fernandez WG et al.[8] and Bagley WH et al.,[9] in our study, 19 (73%) patients had values >5000 U/L, who had presented with AKI.
Interestingly, our study showed an almost equal number of patients with rhabdomyolysis-associated AKI due to nontraumatic causes (53.8%).[10] Seizures cause rhabdomyolysis due to extreme muscle activity, leading to ATP demand-supply mismatch, which culminates in disruption of sarcolemmal membranes.[11] Wasp sting usually causes release of phospholipase and mellitin at the site of sting and can lead to rhabdomyolysis.[12] Gloriosa superba is a wild plant which contains the alkaloids colchicine, gloriosine, superbine, and salicylic acid. It can result in hypotension, volume depletion, rhabdomyolysis, and multiorgan failure. However, it is possible that colchicine may have a direct toxicity on the proximal renal tubules. Anecdotal reports of severe intoxication that demanded renal replacement therapy are available in the literature.[13,14]
The metabolic complications of rhabdomyolysis include hyperkalemia, hyperuricemia, and hypocalcemia, which have to be addressed during treatment.[15] In addition to clinical and laboratory evidence of AKI in rhabdomyolysis, renal biopsy may be done in order to look for signs of irreversible damage. The principal step in the management includes early, aggressive yet careful resuscitation with fluids. Though administration of sodium bicarbonate for the purpose of urinary alkalinization did not show encouraging results in other studies, 15.5% of patients in the nontraumatic category benefited from forced alkaline diuresis, which may be because of early and prompt intervention in the absence of anuria or volume overload in these patients.[16] In our study, the mortality rate due to rhabdomyolysis-associated AKI was around 15.4%; however, it is limited by a small sample size.[12,17]
To conclude, rhabdomyolysis-associated AKI can result from a variety of clinical conditions. Thus, the treating physician should be aware of this wide range of causes including physical assault and other nontraumatic etiologies such as toxins, which are unique to India. A long-term follow-up of these patients should be helpful in identifying the proportion of chronic kidney diseases that result from rhabdomyolysis-associated acute kidney injury.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication:Acute kidney injury. Ann Intensive Care. 2013;3:8.
- [Google Scholar]
- Bench-to-bedside review:Rhabdomyolysis–an overview for clinicians. Crit Care. 2004;9:1-2.
- [Google Scholar]
- Clinical profile and outcome of pigment-induced nephropathy. Clin Kidney J. 2018;11:348-52.
- [Google Scholar]
- Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Inter. 1996;49:314-26.
- [Google Scholar]
- Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis:Implications for follow-up. Crit Care Med. 2002;30:2212-5.
- [Google Scholar]
- Factors predictive of acute renal failure and need for hemodialysis among ED patients with rhabdomyolysis. Am J Emerg Med. 2005;23:1-7.
- [Google Scholar]
- Etiological spectrum and histopathological diagnosis of rhabdomyolysis associated myoglobin cast nephropathy in South India. Indian J Nephrol. 2021;31:22-6.
- [Google Scholar]
- Rhabdomyolysis following status epilepticus with hyperuricemia. Medicine. 2018;97:e11281.
- [Google Scholar]
- Colchicine overdose-induced acute renal failure and electrolyte imbalance. Renal Fail. 2007;29:367-70.
- [Google Scholar]
- Rhabdomyolysis:Historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749-56.
- [Google Scholar]
- Prevention of acute kidney injury by intravenous sodium bicarbonate:The end of a saga. Crit Care. 2014;18:1-2.
- [Google Scholar]







