Translate this page into:
Rituximab in the treatment of refractory late acute antibody-mediated rejection: Our initial experience
Address for correspondence: Dr. S. B. Raju, Department of Nephrology, Nizam's Institute of Medical Sciences, Hyderabad - 500 082, Telangana, India. E-mail: sreebhushan@hotmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Antibody-mediated rejection (AMR) is not uncommon after renal transplantation and is harder to handle compared to cell-mediated rejection. When refractory to conventional therapies, rituximab is an attractive option. This study aims to examine the effectiveness of rituximab in refractory late acute AMR. This is a retrospective study involving nine renal transplant recipients. Four doses of rituximab were administered at weekly interval for 4 weeks, at a dose of 375 mg/m2. The mean age of patients was 35.3 ± 7.38 years. The median period between transplantation and graft dysfunction was 30 ± 20 months. Mean serum creatinine at the time of discharge after transplantation and at the time of acute AMR diagnosis was 1.14 ± 0.19 mg/dl and 2.26 ± 0.57 mg/dl, respectively. After standard therapy, it was 2.68 ± 0.62 mg/dl. One patient died of Pseudomonas sepsis and three patients progressed to end-stage renal disease (ESRD). Four biopsies showed significant plasma cell infiltrations. Mean serum creatinine among non-ESRD patients at the end of 1 year progressed from 2.3 ± 0.4 to 3.8 ± 1.2 mg/dl (P value 0.04). eGFR prior to therapy and at the end of 1 year were 34.4 ± 6.18 and 20.8 ± 7.69 ml/min (P value 0.04), respectively. Only one patient showed improvement in graft function in whom donor-specific antibody (DSA) titers showed significant improvement. Rituximab may not be effective in late acute AMR unlike in early acute AMR. Monitoring of DSA has a prognostic role in these patients and plasma cell rich rejection is associated with poor prognosis.
Keywords
Donor-specific antibody
late acute antibody mediated rejection
plasma cell rich rejection
rituximab
Introduction
Acute antibody-mediated rejection (AMR) is characterized by acute graft dysfunction, histologic evidence of acute tissue injury, deposition of C4d in peritubular capillaries, and the presence of donor-specific antibodies (DSAs).[1234] Acute AMR can be arbitrarily divided into early (occurring within 6 months of renal transplantation) or late (occurring after 6 months of renal transplantation).[5]
An accurate diagnosis of AMR in allograft dysfunction is important since the management is different as compared to cell-mediated rejection and it needs to be treated aggressively. In the last decade, our knowledge about AMR, both acute and chronic, has greatly evolved[67] as a result of remarkable improvements in the technology of anti-human leukocyte antigen antibody detection. The concept of AMR is now well defined,[89] with specific diagnostic criteria laid down by the Banff group and with regular updates of the Banff classification.[24]
International guidelines suggest the use of plasma exchange (PE), intravenous immunoglobulin (IVIG), rituximab, or lymphocyte-depleting antibodies for acute AMR.[10] Rituximab is an attractive option when it is refractory to conventional therapies such as pulse steroids, IVIGs, and PE. The effectiveness of rituximab in early AMR has been emphasized in literature.[11] Rituximab is a chimeric antibody recognizing the cell surface marker CD20, which is expressed at most stages of B-cell development except the very early stages but not on plasma cells.[12] However, its role in late acute antibody-mediated rejection is not well studied, especially in the Indian scenario. In this study, we put forward our experience in treating refractory late AMRs with rituximab.
Materials and Methods
This was a retrospective study involving 9 patients diagnosed with late acute AMR between 1st August 2013 and 31st July 2014, which were resistant to standard therapy (steroid/PE/IVIG). All these patients received rituximab as per protocol and the 1 year outcome of graft function; graft survival and patient survival were evaluated.
Inclusion criteria
Patients who were diagnosed to have acute AMR according to the Banff 07 update[2] were included in the study.
-
AMR was defined as a triad involving the presence of DSA, positive C4d-staining of the biopsy in peritubular capillaries and histopathological evidence of antibody-mediated injury (glomerulitis, peritubular capillaritis, and arteritis)
-
Acute AMR of late onset (occurrence of AMR after 6 months of transplantation)
-
AMR refractory to standard treatment, defined as the deterioration of graft function after receiving 1 gram of methyl prednisolone for 3 consecutive days followed by 7 sessions of PE and 7 doses of low dose IVIG on alternate days
-
Patients who were willing to receive rituximab after ruling out contraindications for rituximab administration.
DSA mean fluorescence intensity (MFI) values >500 were considered significant. We included only those biopsies with diffuse C4d in peritubular capillaries detected by immunohistochemistry and those showing features of glomerulitis plus endothelitis plus peritubular capillaritis without any evidence of chronicity.
PE was done with 20% salt-poor albumin as replacement fluid and 1.5 times the plasma volume was replaced. Prothrombin time and activated partial thromboplastin time was monitored.
Every session of PE was followed by low dose IVIG (100 mg/kg/dose) on the next day. After administration of three doses of one gram of methyl Prednisolone for three consecutive days, oral Prednisolone was started at a dose of 20 mg/day that is tapered to 10 mg/day over 3 months. Mycophenolate mofetil sodium was increased to 720 mg twice daily and continued at the same dose in all patients, and it was discontinued at the time of initiation of dialysis in those patients who reached end-stage renal disease (ESRD). Tacrolimus dose was not changed as serum drug levels were within normal range and continued in all patients and it was discontinued at the time of initiation of dialysis in those patients who reached ESRD.
A total of eleven patients met the diagnostic criteria for late acute AMR in the study period. Two patients were not willing to receive rituximab, after explaining the protocol and complications of rituximab and hence were not included in the study.
After obtaining the informed consent, nine patients were initiated on the rituximab protocol. All the baseline characteristics were recorded, as shown in Table 1. Four doses of rituximab were administered at weekly intervals for 4 weeks at a dosage of 375 mg/m2. We evaluated the graft survival, patient survival and graft function at the end of 1 year along with other demographic features. Graft function and graft survival were monitored by serum creatinine and GFR measured by the MDRD formula.

Statistical analysis
All results were expressed as mean ± standard deviation for continuous variables. Student's t-test was used to compare graft function before and after initiation of rituximab. P < 0.05 was considered significant.
Results
In our study, the mean age of patients was 35.3 ± 7.38 years. Seven of nine patients were men. Average posttransplant duration prior to rejection was 30 ± 20 months. All were live related transplants, and none of the patients received induction therapy. Mean serum creatinine at the time of discharge after transplantation and at the time of acute AMR diagnosis was 1.14 ± 0.19 mg/dl and 2.26 ± 0. 57 mg/dl, respectively.
After standard therapy, it was 2.68 ± 0.62 mg/dl (2.3 ± 0.4 mg/dl in patients who survived and did not reach ESRD at the end of 1 year). All the patients received rituximab according to the dose mentioned. Mean GFR measured by the MDRD formula before initiation of rituximab was 29.55 ± 7.76 ml/min (34.4 ± 6.18 ml/min in those patients who survived and did not reach ESRD at the end of 1 year). Five of these patients had a history of noncompliance and all to mycophenolate mofetil sodium. Renal biopsies of four patients showed rich plasma cell infiltrate in the interstitium and were classified as plasma cell rich-AMR.
Follow-up
Three patients progressed to ESRD at the end of 4 months, 6 months and 10 months and are on maintenance hemodialysis (MHD) [Table 2]. One patient died of Pseudomonas sepsis after 2 months of therapy. Serum creatinine prior to sepsis episode was 4.2 mg/dl and at the time of death was 5.1 mg/dl. Only one patient showed improvement in graft function, with a stable creatinine of 1.9 mg/dl, whereas, in the remaining patients graft function worsened over 1 year. Assessment of graft function in 5 patients who were alive and did not progress to ESRD also showed deterioration at the end of 1 year, which is statistically significant. The mean serum creatinine progressed from 2.3 ± 0.4 to 3.8 ± 1.2 mg/dl (P value 0.04). Mean GFR, measured by the MDRD formula before initiation of rituximab and, at the end of 1 year was 34.4 ± 6.18 ml/min and 20.8 ± 7.69 ml/min (P value 0.04). DSA Class I antibody was positive in one patient, Class II in six patients and both Class I and Class II were positive in two patients. At the end of 1 year, only one patient showed negative DSA titers and, this was the only patient who showed improvement in graft function (patient 1).
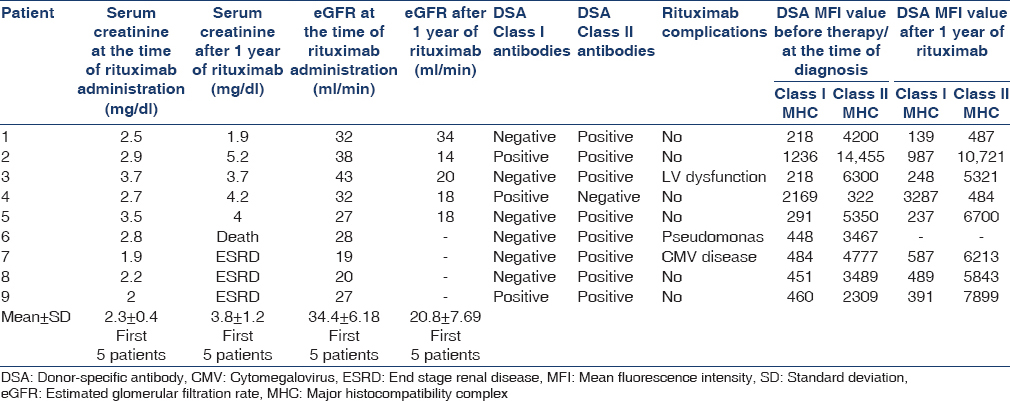
Of four patients who had plasma cell rich rejection, three had graft loss. Three patients developed complications related to rituximab such as pseudomonas sepsis (patient died), CMV disease, and cardiac dysfunction.
Discussion
Acute AMR can be early (occurring within 6 months of renal transplantation) or late (occurring more than 6 months after renal transplantation). The beneficial role of rituximab is well documented in few case series/case reports. However, the majority of these studies are of early acute AMR. In the present study, we examined the role of rituximab in late acute AMR patients.
Becker et al. used a single dose of rituximab to treat 27 patients with AMR.[13] At a mean of 605 days of follow-up, only three grafts were lost to rejection. However, the mean duration of onset of rejection after transplantation in this study was 127 days.
Faguer et al. treated eight patients with four doses of rituximab along with PE and reported 81% graft survival at 20 months.[14] However, the mean duration of onset of rejection after transplantation in this study was 45 days.
Kaposztas et al. published their experience with the use of rituximab in combination with PE. Twenty-six patients were treated with rituximab plus PE and IVIG.[15] The graft outcomes were compared to historical controls who had received PE ± IVIG alone. The 2 years graft survival with rituximab plus PE was at 90%, which, is significantly better when compared to the 60% survival in the PE cohort. The mean duration of onset of rejection after transplantation in this study was 23 days.
Lefaucheur et al. compared the use of 2-week doses of rituximab along with high-dose IVIG and PE with historical controls, who received high-dose IVIG alone and reported a 91.7% graft survival but, it is 50% in high-dose IVIG alone group.[16] Mean duration of onset of rejection after transplantation in this study was 15 days. To the best of our knowledge, this is the first study where the role of rituximab in late acute AMR is assessed. In our study, the mean duration of onset of rejection was 30 months, which, is really high when compared to the other studies.
Contrary to the previous reports, our experience with rituximab showed no beneficial effect. This could be due to the delay in the detection of late acute AMR as patient monitoring was not as rigid as in early postoperative period, and all of them were asymptomatic.
At least five patients had a history of drug noncompliance. Morrissey et al. analyzed the importance of noncompliance in 87 cases of late acute AMR and concluded that noncompliance is a significant factor.[17] In our patients too, noncompliance was a significant contributing factor. We found that four patients had plasma cell rich rejection and three of them progressed to ESRD, which suggests that plasma cells may have a significant role in the rejection. This needs to be addressed in larger patient populations.
In our series, only one patient showed improvement in graft function, whereas, in all the remaining patients, graft function deteriorated; 3 of 8 patients reached ESRD and were put on MHD. DSA mean MFI decreased to <500 in the stable patient and in the remaining patients it is decreased but still MFI was more than 2000. This reflects that DSA monitoring has prognostic value. A study conducted by Lefaucheur et al. also showed that DSA monitoring is useful in monitoring of acute AMR.[16] When we analyzed the data with the Student's t-test in those five live patients who did not progress to the stage of ESRD, there is a statistically significant deterioration of graft function indicating that rituximab is not effective in this group of patients. Though application of Student's t-test for such a small sample size may not be appropriate, we believe that rituximab may not be effective as, out of nine patients, three patients progressed to ESRD and five patients showed deterioration of renal function.
Three latest studies showed that the addition of rituximab to standard therapy was not associated with the superior graft function.
1-year results were recently reported from a phase III, multicenter, randomized, placebo-controlled trial[18] (RITUX ERAH) that examined the effect of rituximab (combined with PE, IVIG, corticosteroids, tacrolimus and mycophenolate mofetil) on a composite measure of graft loss or absence of improvement of renal function at day 12, in 38 patients with biopsy-proven acute ABMR.
In this study, out of 38 patients, they administered a single dose of rituximab to 19 patients. 42% of cases have been of late acute AMR. A composite measure of graft loss or absence of improvement of renal function frequency at day 12 was 52.6% and 57.9% in the rituximab and placebo groups, respectively (P = 0.74) with no advantage of rituximab over control for the graft loss or renal function outcome. At the end of 1 year, both groups showed improvement in serum creatinine, histological parameters, and DSA levels; however, in the rituximab group, there is no additional benefit when compared to additional therapy and this trial concluded that the addition of rituximab to standard therapy of PE, IVIG, and steroids provides no additional benefit.
Gupta et al.[5] published their data in 23 cases of late acute AMR. Out of 23 patients, 18 patients received rituximab in addition to PE/IVIG and steroids and assessed serum creatinine, histologic improvement, and DSA titers. Initially, there was a marginal benefit observed with respect to serum creatinine but after follow-up of 71 days (43-802 days), worsening of serum creatinine, lack of histologic response and high DSA levels were observed and concluded that rituximab was not effective in late AMR.
Gulleroglu et al.[19] studied the effect of rituximab in 3 pediatric cases of refractory late acute AMR by administering 2–4 doses of rituximab (375 mg/m2). At the end of 1 year, two grafts were lost and concluded that addition of the rituximab to standard therapy was ineffective.
Having a small sample size in our study is definitely an important limitation along with a retrospective analysis of the data. A repeat biopsy at the end of the study would have been provided prognostic value which was not done in our study. We need randomized control trials with a larger sample size to confirm these results.
Conclusion
Rituximab may not be effective in late acute AMR unlike, in early acute AMR. Monitoring of DSA has a prognostic role in these patients and plasma cell rich rejection is associated with poor prognosis. These things should be confirmed by further studies involving larger sample size.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are thankful to Dr. Ch Ved Prakash Rao for helping in preparing this manuscript.
References
- Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753-60.
- [Google Scholar]
- Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464-71.
- [Google Scholar]
- Late antibody-mediated rejection in renal allografts: Outcome after conventional and novel therapies. Transplantation. 2014;97:1240-6.
- [Google Scholar]
- Antibody-mediated rejection in renal allografts: Lessons from pathology. Clin J Am Soc Nephrol. 2006;1:415-20.
- [Google Scholar]
- The spectrum of antibody-mediated renal allograft injury: Implications for treatment. Am J Transplant. 2008;8:1367-73.
- [Google Scholar]
- Antibody-mediated rejection criteria – An addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708-14.
- [Google Scholar]
- National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4:1033-41.
- [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1-155.
- [Google Scholar]
- Antibody-mediated rejection in kidney transplantation: A review. J Transplant 2012 2012:193724.
- [Google Scholar]
- Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant. 2004;4:996-1001.
- [Google Scholar]
- Rituximab therapy for acute humoral rejection after kidney transplantation. Transplantation. 2007;83:1277-80.
- [Google Scholar]
- Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant. 2009;23:63-73.
- [Google Scholar]
- Comparison of combination plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9:1099-107.
- [Google Scholar]
- Factors contributing to acute rejection in renal transplantation: The role of noncompliance. Transplant Proc. 2005;37:2044-7.
- [Google Scholar]
- One year results of the effects of rituximab on acute humoral rejection in renal transplantation: RITUX ERAH, a multicenter randomized placebo controlled trial. Am J Transplant. 2013;13(Suppl 5):1-9. [Abstract #266]
- [Google Scholar]
- Antibody-mediated rejection and treatment in pediatric patients: One center's experience. Exp Clin Transplant. 2013;11:404-7.
- [Google Scholar]







