Translate this page into:
Role of C4d in the Diagnosis and Prognosis of Native Renal Diseases
Address for correspondence: Dr. Varsha Kumar, Department of Pathology, M.L.N. Medical College, Prayagraj – 211 001, Uttar Pradesh, India. E-mail: E-mail: drvarshakumar1@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
C4d is a biomarker of the complement cascade and has a primary role in the diagnosis of antibody-mediated rejection in solid organ transplantation. The present study was undertaken to investigate the role of C4d in the diagnosis and prognosis of native renal diseases.
Methods:
An observational cross-sectional study was conducted in the Department of Pathology from September 2017 to September 2019. In this study, we applied C4d staining by immunohistochemistry in 51 native renal biopsies. Semiquantitative scoring was done on the basis of intensity of C4d staining along the glomerular capillary wall (0–3) and mesangium (0–3), tubules (0–3), and arteries (0–3). These individual scores were added to get the total C4d score (0–12) which was correlated with chronicity index, serum urea and creatinine levels. Glomerular C4d score was correlated with 24 h urinary protein as well as with immunofluorescence deposition of immunoglobulins and complements.
Results:
We found a linear positive correlation (P < 0.05) between the total C4d score and serum creatinine; tubular C4d score and serum creatinine; and glomerular C4d score along capillary wall and 24 h urinary protein. A positive correlation (P < 0.05) was found between glomerular C4d score along the capillary wall with immunofluorescence deposits of immunoglobulins and complements, suggesting the efficacy of C4d as a surrogate marker in the diagnosis of native renal diseases.
Conclusions:
C4d deposition is associated with a poor prognosis in renal diseases and an accelerated deterioration of renal function. It also plays a role as a surrogate marker in diagnosis of native renal diseases.
Keywords
C4d
diagnosis
prognosis
surrogate marker
Introduction
C4d is a footprint of antibody-mediated cell injury. It has a role in the diagnosis of antibody-mediated rejection (AMR) in solid organ transplantation, since its incorporation in Banff classification in 2003. Identification of C4d along the peritubular capillaries is identified as evidence of AMR. The classical pathway is activated by the binding of the complement C1 complex to the antigen–antibody complex, which is accompanied by the cleavage of C4 into C4a and C4b, after which C4b is cleaved into C4c and C4d.[123456] This cleavage provides a covalent bond that helps C4d to anchor to nearby cells where immune complexes are deposited. Antibodies dissociate naturally because of relatively weak hydrostatic and Van der Waals forces between antigens and antibodies, whereas the covalent bond of C4d has a much longer half-life. For this reason, C4d serves as a footprint of antibody mediated tissue injury.
It is well known that complement activation by immune complex, through the classical pathway, plays a major role in the pathogenesis of some glomerulonephritis.[6789101112] C4d is also generated by activation of the lectin pathway. Activation of the lectin pathway of complement is associated with more severe renal damage. Furthermore, C4d staining is an inexpensive and easy method for the investigation of renal biopsies.[1234513]
Very few studies are available focusing on the role of C4d in entities such as lupus nephritis, IgA nephropathy, and membranous nephropathy. There is paucity of data from Indian Subcontinent on an extensive search for literature. Hence, this study was undertaken in an attempt to observe the pattern of C4d deposition in various native renal diseases and draw correlation with various biochemical parameters.
Materials and Methods
An observational cross-sectional study was conducted in the Department of Pathology from September 2017 to September 2019. A total of 51 renal biopsies from patients presenting with nephrotic syndrome, nephritic syndrome, isolated hematuria, azotemia were obtained from the Department of Medicine and Paediatrics. USG-guided renal biopsy was performed and fixed in 10% formalin for light microscopic evaluation.
Renal biopsies received in normal saline were transferred to vial containing isopentane. Snap freezing was performed by further suspending the vial in liquid nitrogen. Crytostat sections were cut and subjected to immunofluorescence staining using FITC-conjugated antibodies IgG, IgA, IgM, C3, C1q, kappa and lambda (Bio SB). Pattern and intensity of deposits were recorded.
Renal biopsies with less than ten glomeruli were excluded from the study. Biopsies were stained with hematoxylin and eosin (H and E), Periodic Acid–Schiff (PAS), Masson's trichrome, and silver methenamine.
Immunohistochemical analysis of C4d
C4d immunohistochemical staining was performed on 3–4-μ-thick deparaffinized and rehydrated sections of formaldehyde fixed renal biopsy, using rabbit polyclonal C4d antibody (Bio SB). Antigen retrieval was performed in advance of slide treatment by Tinto retriever pressure cooker (20 min at 106–110°C in Tris Buffer, pH 10.0) and cooling to room temperature. The slides were incubated in primary antibody for 60 min, followed by incubation in HRP polymer for 45 min, and chromogen DAB for 8–10 min. Each step was followed by washing thrice in PBS for 2–3 min each.
Biopsies of known case of membranous nephropathy and minimal change disease (MCD) were taken as positive and negative controls respectively and were included with each batch of staining.
Semiquantitative scoring to evaluate the total chronicity index score was done according to modified Banff Criteria (2013) [Table 1]. Semiquantitative scoring for C4d deposition was done based on intensity of C4d staining in the various compartments of renal biopsy. Staining was evaluated in capillary walls (0–3+), mesangium (0–3+), tubular epithelium (0–3+), and arteries C4d (0–3+). Images were captured by a Zeiss microscope (Primo Star) using TC Capture software [Table 2]. These individual scores were added to get the total C4d score (0–12) and were correlated with the total chronicity index score, semiquantitative chronicity index score of interstitial fibrosis and tubular atrophy. The total C4d score was correlated with biochemical parameters like serum urea and creatinine. The glomerular capillary wall C4d score was correlated with 24 h urinary protein as well as with immunofluorescent deposits of immunoglobulins and complements. IF staining for C4d could not be done due to financial constraints, hence, only IHC was used to compare and correlate the results. Statistical analysis was done by Pearson correlation coefficient to determine the P value. Ethical clearance from institutional ethics committee was obtained.
| Morphology | Score 0 | Score 1 | Score 2 | Score 3 |
|---|---|---|---|---|
| Glomerulosclerosis* | Absent | <25% | 25-50% | >50% |
| Tubular Atrophy | No T.A | <25% | 25-50% | >50% |
| Interstitial Fibrosis | <5% | 6-25% | 26-50% | >50% |
| Vascular changes | No fibrous intimal thickening | <25% | 26-50% | >50% |
| C4d staining | Scores |
|---|---|
| No staining | 0 |
| Mild staining | 1+ |
| Moderate staining | 2+ |
| Intense staining | 3+ |
Results
Clinicopathological spectrum of renal diseases
A total of 51 biopsies were included in the study. Mean age of study population was 28.41 ± 18.12 years (1.25–69 years). Male to female ratio was 1.7:1. Most common cause of nephrotic syndrome (52.2%) was membranous nephropathy (19.6%) followed by MCD (15.2%) and focal segmental glomerulosclerosis (10.9%).
Among nephritic syndrome (19.6%), the leading cause was lupus nephritis (8.7%) followed by proliferative glomerulonephritis (6.5%) and IgA nephropathy (4.4%). Tubulo interstitial disease (13.0%), diffuse global glomerulosclerosis (4.4%), paraprotein-associated diseases (2.2%), diabetic nephropathy (2.2%), thrombotic microangiopathy (2.2%), and resolving PIGN (4.4%) represented the remaining spectrum.
Pattern of C4d deposition in renal diseases
MCD accounted for 15.2% cases (7/46). Mean age was 7.78 ± 4.30 years (2–14 years). Male: female ratio was 6:1. All patients presented with nephrotic syndrome. Urinalysis revealed bland sediments. Diagnosis of MCD was rendered in histomorphologically normal appearing glomeruli with no deposition of immunoglobulins and complements on immunofluoresecnce. Likewise, C4d immunostaining was also negative [Figure 1a, b, c; Table 3].

- a. Unremarkable glomerulus in MCD (PAS, 400X) b. Negative immunofluorescence in MCD (IF, 100X) c. No C4d deposits along the capillary wall in MCD (IHC, 400X) d. Thickened capillary wall in membranous nephropathy (PAS 400X) e. Fine granular deposits of IgG along the capillary wall in MN (IF, 400X) f. Intense staining of C4d along the capillary wall and moderate staining of tubules in MN (IHC, 400X) g. Segmental sclerosis in FSGS (PAS, 400X) h. Segmental sclerosis in FSGS (IF, 400X) i. C4d deposition in tip lesion variant of FSGS (IHC, 400X)
| Diagnosis | Immunofluorescence | IHC | |
|---|---|---|---|
| Ig | C3 | C4d | |
| MCD | 0 | 0 | 0 |
| MN | 4+ cw (IgG) | 2-3+ cw | 2-3+ cw |
| FSGS | 2-3+ | 0 | 0-1+ sclerosed |
| Segmental (IgM) | Segment | ||
| MPGN | 2+ cw and | 3+ cw | 2+cw |
| Mes (IgG) | and mes | ||
| Amyloidosis | 0 | 0 | 3 + (mes) |
| LN (class IV) | 4+ cw and Mes | 4+ cw and mes | 3+cw |
| Proliferative GN | 3+cw (IgG) | 0 to 3+ cw | 2+cw |
| Mes PGN | 3+ mes (IgA) | 2+ mes | 2+mes |
| MGRS | 3+ cw (IgG) | 3+ cw | 2+cw |
| DGGS | - | - | - |
cw=capillary wall, mes=mesangial, IHC=immunohistochemistry. MCD, Minimal change disease; MN, Membranous nephropathy; FSGS, Focal segmental glomerulosclerosis; MPGN, Membranoproliferative glomerulonephritis; LN, Lupus nephritis; Mes PGN, Mesangial proliferative glomerulonephritis; MGRS, Monoclonal gammopathy of renal significance, DGGS, Diffuse global glomerulosclerosis
Membranous nephropathy (MN) accounted for 19.6% cases (9/46). Mean age was 28.77 ± 17.7 years (10–69 years). Male: female ratio was 1.25:1. All patients presented with nephrotic syndrome. Light microscopy revealed thickening of glomerular capillary walls, with demonstration of epimembranous argyrophilic spikes. In majority of cases, tubulointerstitial compartment and blood vessels were largely unremarkable. One case displayed unremarkable glomeruli on light microscopy. On immunofluorescence, fine granular deposits of IgG and C3 were noted along the capillary walls. Hence, diagnosis of early MN was made based on immunofluorescence. All cases displayed C4d deposition along capillary walls [Figure 1d, e, f; Table 3].
FSGS accounted for 10.9% cases (5/46). Mean age was 26.85 ± 21.87 years (1.25–65 years). Male: female ratio was 4:1. Majority presented with nephrotic syndrome. Light microscopy revealed FSGS with unremarkable tubulointerstitial compartment. Nonspecific trapping of IgM and C3 was seen in the affected sclerosed segment on IF. [Figure 1g, h]. C4d immunostaining showed variable positivity in the mesangium.[Table 3] In one case, synechiae formation was noted between the segment of glomerular tuft with proximal tubular epithelium. On immunohistochemistry, C4d deposits were noted in the sclerosed segment with synechiae formation with the tubular pole. Diagnosis of “Tip lesion”—variant of FSGS—was rendered in this case [Figure 1i].
MPGN accounted for 4.4% cases (2/46). Mean age was 27.5 ± 6.5 years (21–34 years). Male preponderance was noted. All patients presented with nephrotic-nephritic syndrome. On histopathology, lobulation of glomerular tuft, increase in mesangial cellularity, and thickening of capillary walls with splitting of the basement membrane was demonstrated. C4d immunohistochemical staining along the glomerular capillary wall correlated with immunofluorescence deposition of IgG and C3 along capillary walls. Mesangial C4d staining was variable [Figure 2a, b; Table 3].
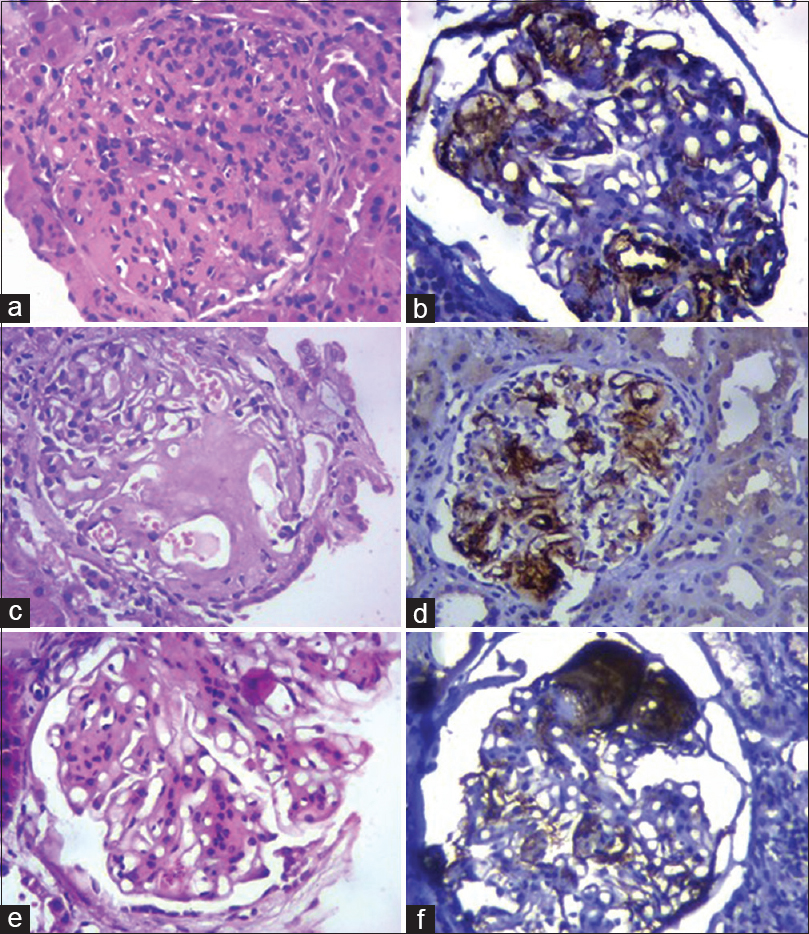
- a. MPGN showing global endocapillary proliferation (H and E, 400X) b. C4d deposits along the thickened glomerular capillary wall in MPGN (IHC, 400X) c. Enlargement of glomerulus with deposition of amorphous eosinophilic material in the mesangium and along capillary walls in amyloidosis. (H and E, 400X) d. Deposition of C4d in mesangium and along capillary walls in amyloidosis (IHC, 400X) e. Nodular diabetic glomerulosclerosis showing Kimmelstiel–Wilson nodule (H and E, 400X) f. Bright deposition of C4d in diabetic nodule (IHC, 400X)
In amyloidosis, histopathological examination revealed enlargement of glomeruli with focal deposition of amorphous eosinophilic material in the mesangium and along capillary walls. The amorphous deposits were weakly PAS positive. Demonstration of congophilic deposits under polarizing microscope confirmed the diagnosis. Strong C4d mesangial staining was seen in amyloidosis. No such deposits of immunoglobulins and complements were observed on immunofluorescence [Figure 2c, d; Table 3]
Diabetic nodular glomerulosclerosis revealed nodule formation in the glomeruli and showed strong staining of the nodule for C4d. This staining pattern did not conform with the immunofluorescent staining of immunoglobulins and complements. [Figure 2e, f; Table 3] Lupus nephritis showed full house pattern on IF, along capillary walls, and mesangium in class III and IV Lupus nephritis, with only mesangial deposits in Class I LN. On immunohistochemical staining for C4d, a similar pattern of deposition was noted.
There were 6.5% cases (3/46) in the Proliferative GN group. Mean age was 19 ± 8.28 years (10–30 years) and male: female ratio was 2:1. All patients presented with nephritic syndrome. On histology, there was diffuse enlargement of glomeruli with endocapillary proliferation. Typically, 33.3% (1/3) showed crescent formation in glomerulus and another patient 33.3% (1/3) presented with postinfectious glomerulonephritis. Proliferative glomerulonephritis showed bright staining of C4d along the capillary wall similar to immunofluorescence deposits of IgG and complements. Crescentic glomerulonephritis denoted bright IF deposits of IgG and C3 along with IHC staining for C4d along the glomerular capillary wall, ascribing to immune complex mediated crescentic glomerulonephritis.
On immunofluorescence, MesPGN demonstrated IgA dominant deposits in the mesangium. C4d mesangial immunohistochemical staining was noted in all the cases. These cases had a significant component of tubular atrophy and interstitial fibrosis, ascribing it HAAS Class V.
Glomerular C4d along the capillary wall showed a significant positive correlation with immunofluorescence deposits of immunoglobulins and complements (P < 0.05), suggesting the efficacy of C4d as a surrogate marker of immunofluorescence in diagnosis of native renal diseases. [Figure 3, Table 4]
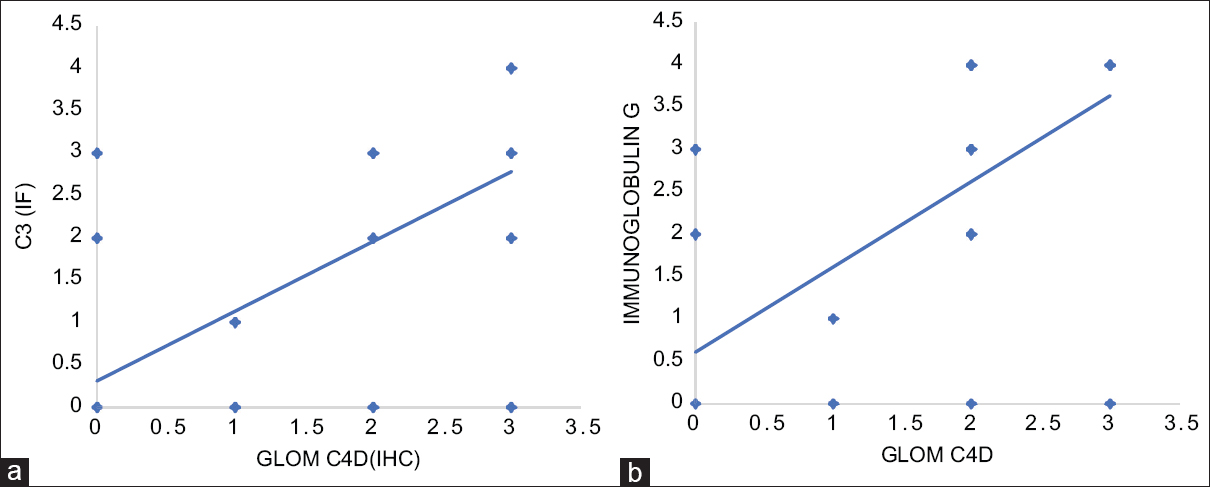
- a. Correlation between the glomerular C4d capillary wall score and C3 (IF) (P < 0.001) b. Correlation between the glomerular C4d capillary wall score and IgG (IF). (P < 0.001)
| C4d score | Variables | Correlation coefficients |
|---|---|---|
| Total C4d score | Total CI | R=0.5889, P<0.001 |
| Total C4d score | IF | R=0.4168, P=0.004 |
| Total C4d score | TA | R=0.5651, P<0.001 |
| Total C4d score | S. Creatinine | R=0.3214, P=0.03 |
| Total C4d score | 24 h U. Protein | R=0.0684, P=0.65 |
| Total C4d score | S. Urea | R=0.1395, P=0.36 |
| Tubular C4d score | S. Creatinine | R=0.3255, P=0.02 |
| Tubular C4d score | S. Urea | R=0.1955, P=0.18 |
| Glom C4d (cw) | 24 h U. Protein | R=0.3327, P=0.02 |
| Glom C4d (mes) | 24 h U. Protein | R = -0.2338, P=0.12 |
| Glom C4d (cw) | C3 | R=0.6652, P<0.001 |
| Glom C4d (cw) | IgG | R=0.6997, P<0.001 |
Correlation of C4d with other biochemical parameters
A linear positive correlation was found between the total C4d score and the total chronicity index, which incorporated glomerulosclerosis, IFTA, and vascular changes. (P < 0.05). Similarly, a linear positive correlation was noted between the total C4d score and the interstitial fibrosis and tubular atrophy score (P = 0.004 and < 0.001, respectively) [Table 4].
A positive linear correlation between the total C4d score and serum creatinine was demonstrated. The deposition of C4d in various compartments was associated with poor renal function. (P < 0.05) [Table 4; Figure 4]. The tubular C4d score and serum creatinine followed similar trends (P < 0.05) [Figure 4].

- a. Correlation between the total C4d score and S. Creatinine. (P = 0.03) b. Correlation between the tubular C4d score and S. Creatinine. (P = 0.02) c. Correlation between the glomerular C4d capillary wall score and 24 h urinary protein. (P = 0.02) d. Correlation between the glomerular C4d mesangial score and 24 h urinary protein. (P = 0.12)
The glomerular C4d score along the capillary wall correlated with 24 h urinary protein (P < 0.05). Thus, it denotes the role of C4d as a footprint of complement activation in glomerular damage [Figure 4].
Discussion
C4d is a degradation product of complement cascade and its expression is recognized as a footprint of complement activation via the classical and lectin pathways. In renal transplantation, the presence of C4d in peritubular capillaries with morphological evidence of allograft damage and donor-specific antibody is diagnostic of acute AMR.
The role of C4d as a diagnostic marker has been studied recently in various immune complex-mediated glomerulonephritis. In our study, MCD showed no deposits of immunoglobulins and complements on immunofluorescence. In concordance, C4d immunostaining along the glomerular basement membrane was negative [Table 3]. This finding was concordant with the study done by Espinosa-Hernández M et al.[10] MCD does not involve complement system and are usually used as control in studying the complement pathways.[14]
Strong C4d immunohistochemical staining was demonstrated along capillary walls in MN. This observation thereby establishes the role of C4d in diagnosing early MN, which morphologically resembles MCD. This finding is in concordance with the study by Espinosa-Hernández M et al.[10] and Val-Bernal JF et al.[15] Rodriguez EF et al.[16] demonstrated C4d as a marker for early recurrent MN.
“Tip lesion” variant of FSGS displayed synechiae formation between segment of glomerular tuft with proximal tubular epithelium. On immunohistochemistry, C4d deposits were noted in the sclerosed segment with synechiae formation with the tubular pole. Rest of the glomeruli were unremarkable. Son MJ et al.[17] demonstrated the deposition of C4d, product of lectin pathway in FSGS, and suggested its role in pathogenesis. The study also documented the expression of C4d to be related to proteinuria.
Mesangial deposition of C4d was observed in amyloidosis kidney and diabetic nodular glomerulosclerosis. Our findings are concordant with the study by Yadav S et al.[18] who observed that 50% cases of diabetic nephropathy demonstrated positive glomerular C4d deposition. In a study by Li XQ et al.[19], the plasma levels of C1q, MBL, Bb, C4d, C3a, C5a, and sC5b-9 were significantly higher in DN patients.
C4d deposition was noted along capillary walls and mesangium in 75% cases of lupus nephritis and all cases of MPGN. Kim MK. et al.[3] with the help of glomerular C4d staining found that the activation of lectin pathway and classical pathway play a crucial role in pathogenesis of lupus nephritis. Martin M et al.[20] studied plasma C4d levels by ELISA in SLE patients and found it significantly raised in lupus nephritis, thus useful in early diagnosis and possibly as a valuable marker for monitoring. C4d deposits along the capillary wall can help to diagnose cases of early membranous nephropathy, lupus nephritis Class I displaying normal light microscopic features.
Mesangial proliferative glomerulonephritis (IgA nephropathy) was found to be associated with tubulointerstitial nephritis. Strong mesangial C4d immunostaining denoting activation of the lectin pathway was noted in our study, similar to the findings by Sethi S et al.[8] Nasri H et al.[21] stated glomerular deposition of C4d is a sign of activation of the lectin complement pathway and was associated with more severe renal damage. Positive C4d staining was significantly associated with clinical/histopathological severity of IgA nephropathy in the study by Baek HS et al.[22] Similar deposition of glomerular C4d in IgA nephropathy was noted in a study done by Pandey S et al.[23]
In monoclonal gammopathy of renal significance, immunofluorescence deposits of IgG and C3 and IHC deposits of C4d were noted along the glomerular capillary wall. Negativity of PLA2R and thrombospondin suggested secondary MN, ruling out idiopathic MN and lupus nephritis, respectively. Heavy chain restriction of IgG4 and IgG1 clinched the diagnosis.
Intensity of glomerular C4d along the capillary wall by immunohistochemistry strongly correlated with immunofluorescence deposits of complement and immunoglobulins and was statistically significant (P < 0.05). This finding is concordant with the study by Kim MK et al.[3]
A positive linear correlation was demonstrated between the total C4d score and serum creatinine levels. The tubular C4d showed a positive correlation with serum creatinine and was statistically significant (P < 0.05). However, this finding was in contrast to the study done by Pandey S. et al.[23]
Statistically significant positive correlation was noted between glomerular C4d deposits and 24 h urinary protein. (P < 0.05). Concordance was found with the study by Maeng YI et al.[12] who found that glomerular C4d deposition was associated with albuminuria (P = 0.004). Likewise, Son MJ et al.[17] documented that C4d expression might be related to the amount of proteinuria rather than the disease type.
Conclusion
C4d deposition in the glomerulus denotes activation of the classical or lectin complement pathway and can act as a surrogate marker in subtyping of various glomerular diseases. C4d staining helps in identifying patients with poor renal function. Given its prognostic importance, staining for C4d might be incorporated into routine analysis of renal biopsies.
Financial support and sponsorship
The study was supported by grants from DHR-MRU 005, KGMU, Lucknow.
Conflicts of interest
There are no conflicts of interest.
References
- Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724-34.
- [Google Scholar]
- Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol. 2012;23:381-99.
- [Google Scholar]
- Pathogenesis and significance of glomerular C4d deposition in lupus nephritis: Activation of classical and lectin pathways. Int J Clin Exp Pathol. 2013;6:2157-67.
- [Google Scholar]
- IgA nephropathy: An update on pathogenesis and classification. J Coll Physicians Surg Pak. 2011;21:230-3.
- [Google Scholar]
- IgA nephropathy: Association of C4d with clinical and histopathological findings and possible role of IgM. Ren Fail. 2015;37:1464-9.
- [Google Scholar]
- Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015;26:1503-12.
- [Google Scholar]
- Mesangial C4d deposition: A new prognostic factor in IgA nephropathy. Nephrol Dial Transplant. 2009;24:886-91.
- [Google Scholar]
- Glomerular and tubular C4d depositions in IgA nephropathy: relations with histopathology and with albuminuria. Int J Clin Exp Pathol. 2013;6:904-10.
- [Google Scholar]
- Nomenclature of the Oxford classification of IgA nephropathy: Do we need to be careful? Kidney Int. 2010;77:74.
- [Google Scholar]
- C4d immunohistochemical staining is a sensitive method to confirm immunoreactant deposition in formalin-fixed paraffin-embedded tissue in membranous glomerulonephritis. Histol Histopathol. 2011;26:1391-7.
- [Google Scholar]
- The pathology and clinical features of early recurrent membranous glomerulonephritis. Am J Transplant. 2012;12:1029-38.
- [Google Scholar]
- Significance of C4d expression in minimal change disease and focal segmental glomerulosclerosis. Int J Clin Exp Pathol. 2016;9:8215-22.
- [Google Scholar]
- Utility of C4D deposits in native renal diseases and relation with disease progression. Indian J Health Sci Biomed Res. 2019;12:50-5.
- [Google Scholar]
- Complement activation in patients with diabetic nephropathy. Diabetes Metab. 2019;45(3):248-253.
- [Google Scholar]
- Plasma C4d as marker for lupus nephritis in systemic lupus erythematosus. Arthritis Res Ther. 2017;19:266.
- [Google Scholar]
- Association of glomerular C4d deposition with morphologic variables of Oxford classification in IgA nephropathy patients; a preliminary study. Immunopathol Persa. 2016;2:e18.
- [Google Scholar]
- Clinical relevance of C4d deposition in pediatric immunoglobulin A Nephropathy. Fetal Pediatr Pathol. 2018;37:326-36.
- [Google Scholar]
- C4d deposition in native kidney disease and its correlation with proteinuria and serum urea/creatinine. Int J Res Med Sci. 2018;6:3935-41.
- [Google Scholar]







