Translate this page into:
SARS-CoV-2 Antibody Seroprevalence and Humoral Response to Vaccination in Patients Undergoing Maintenance Hemodialysis: A Prospective Cohort Study
-
Received: ,
Accepted: ,
How to cite this article: Senthilkumaran G, Rajarathinam VD, Govindarajan S, Jibia VS, Balasubramanian CM, Devaraju PK, et al. SARS-CoV-2 Antibody Seroprevalence and Humoral Response to Vaccination in Patients Undergoing Maintenance Hemodialysis: A Prospective Cohort Study. Indian J Nephrol. 2024;34:129–33. doi: 10.4103/ijn.ijn_7_23
Abstract
Introduction:
COVID-19, caused by SARS-CoV-2, has resulted in significant mortality and morbidity worldwide. Patients of chronic kidney disease who are on maintenance hemodialysis represent a vulnerable population cohort that is susceptible to severe disease. Hence, it is of utmost importance to prioritize vaccination in this population and to assess their response to said vaccination.
Methods:
This prospective analytical study was conducted at the Institute of Nephrology, Madras Medical College, between August 2021 and February 2022. Patients of chronic kidney disease stage 5 dialysis (CKD5D) who were on maintenance hemodialysis and who consented to receive COVID-19 vaccine were studied. Serum samples were obtained before vaccination, ≥28 days after receiving the first dose of the vaccine, and ≥28 days after receiving the second dose. Antibody titers against the spike protein were estimated using the Roche chemiluminescent immunosorbent assay. Patients were grouped into non-responders/seronegative (<0.8 U/ml) and responders/seropositive (≥0.8 U/ml), with a value ≥250 U/ml considered as robust response.
Results:
A total of 96 patients were included. The mean age was 36.70 (±11.53) years and 77.1% of them were male. The median dialysis vintage was 2 (IQR: 0.95–5) years. Twelve patients (9.9%) had a prior COVID-19 infection. Sixty-seven (69.8%) patients had received Covaxin and 29 (30.2%) had received Covishield vaccines. Among the 17 patients who were seronegative at baseline, 4 (23.52%) became seropositive after the first dose of the vaccine, and 11 (64.7%) were seropositive after the second dose, with high titers (“robust response”) achieved in two patients (11.76%). No antibody response, despite two doses of the vaccine, was noted in six patients (35.29%).
Conclusion:
Our study showed a high baseline seropositivity rate, even prior to vaccination, which indicated a high rate of subclinical COVID infection. Among those who were seronegative at baseline, the seroconversion rate after two doses of Covaxin or Covishield was 64.70%.
Keywords
COVID-19
end stage renal disease
humoral response
maintenance hemodialysis
seroprevalence
vaccination
Introduction
The COVID-19 pandemic has had devastating consequences on millions worldwide. Patients with chronic kidney disease (CKD), especially those on maintenance hemodialysis, represent a particularly vulnerable and high-risk population.1,2 Concerted efforts have been made to expedite the approval and roll-out of vaccines for COVID-19 once efficacy has been demonstrated. However, patients with end-stage kidney disease (ESKD) remain underrepresented in these vaccine trials. Furthermore, their ability to mount an immune response may be attenuated owing to their immunocompromised state. In this paper, we report the baseline seroprevalence and the subsequent antibody response to the vaccination of 96 patients on maintenance hemodialysis.
Materials and Methods
Ours was a prospective analytical study conducted at the Institute of Nephrology, Madras Medical College, between August 2021 and February 2022, after obtaining approval from the Institutional Ethics Committee. Patients with CKD stage 5 dialysis (CKD5D) who were on maintenance hemodialysis and who consented to receive either of the two available COVID-19 vaccines (Covaxin [BBV152] or Covishield [SII – ChAdOx1 nCoV-19]) were studied. Covaxin is an indigenous inactivated virus vaccine produced by Bharat Biotech in collaboration with the Indian Council of Medical Research,3 whereas Covishield is an adenoviral vector vaccine developed using technology shared by Oxford University/AstraZeneca and produced by the Serum Institute of India Private Limited.4 Two doses of the vaccine were administered intramuscularly into the deltoid at intervals recommended by national guidelines at the time. Patients with HIV infection and those who were currently on immunosuppressive medication were excluded from our study. Patient-related data, including comorbidities, dialysis details, prior COVID-19 infection, and vaccine-related adverse events, were collected.
Serum samples were collected at three time-points: at baseline, 28 days after the first dose, and 28 days after the second dose of the vaccine. Antibody titers against the spike protein (S) were estimated using the chemiluminescent immunosorbent assay (CLIA). Based on the response obtained, patients were grouped into non-responders (<0.8 U/ml) and responders (≥0.8 U/ml). Values ≥250 U/ml were considered to indicate a robust response and represent high titers.5 At every dialysis visit, patients were screened for symptoms of COVID-19 infection (tested when indicated).
Statistical analysis
Categorical variables were expressed as number and proportion, normally distributed continuous variables were expressed as mean and standard deviation, and non-normally distributed variables were expressed as median and interquartile range. Appropriate tests of statistical significance were used: the Chi-squared test for categorical data, the independent t-test for normally distributed data, and the Mann–Whitney U test for non-normally distributed data. A two-sided P value <0.05 was considered to be statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 28. Factors associated with the development of antibody response were analyzed. These included patient characteristics, disease- and dialysis-related factors such as age, gender, comorbidities, dialysis vintage, dialysis access, and biochemical parameters.
Results
Patient characteristics
Among the 121 patients on maintenance hemodialysis and who were screened, 96 met the inclusion criteria. The mean age of those 96 patients was 36.70 (±11.53) years and 77.1% were male. The median dialysis vintage was 2 (IQR: 0.95–5) years. Six patients were on dialysis after undergoing renal transplant. Twelve patients were documented to have had a prior COVID-19 infection. Other relevant patient characteristics are as described in Table 1.
| Characteristic | All patients (n=96) |
|---|---|
| Age (years) [mean±SD] | 36.70±11.53 |
| Male sex [n (%)] | 74 (77.1%) |
| Body mass index (kg/m2) [mean±SD] | 19.68±3.87 |
| Dialysis vintage (years) [median (IQR)] | 2 (IQR: 0.95–5) |
| Hypertension [n (%)] | 79 (82.3%) |
| Coronary artery disease [n (%)] | 10 (10.4%) |
| Hemoglobin (g/dl) [mean±SD] | 7.98±1.50 |
| Serum calcium (mg/dl) [mean±SD] | 7.91±1.11 |
| Serum phosphorus (mg/dl) [mean±SD] | 4.90±1.44 |
| Serum albumin (g/dl) [mean±SD] | 3.86±0.50 |
| Arteriovenous fistula as dialysis access [n (%)] | 93 (96.9%) |
| Chronic hepatitis B infection [n (%)] | 11 (11.5%) |
| Past or current hepatitis C infection [n (%)] | 18 (18.8%) |
| Documented prior COVID-19 infection [n (%)] | 12 (12.5%) |
SD: Standard deviation, IQR: interquartile range.
Baseline seroprevalence
Of the 96 patients included in the study, 92 had samples drawn prior to vaccination, to assess baseline seroprevalence prior to vaccination. Seropositivity was noted in 75 patients (81.52%), among whom 36 (48%) had high titers (>250 U/ml) [Figure 1].
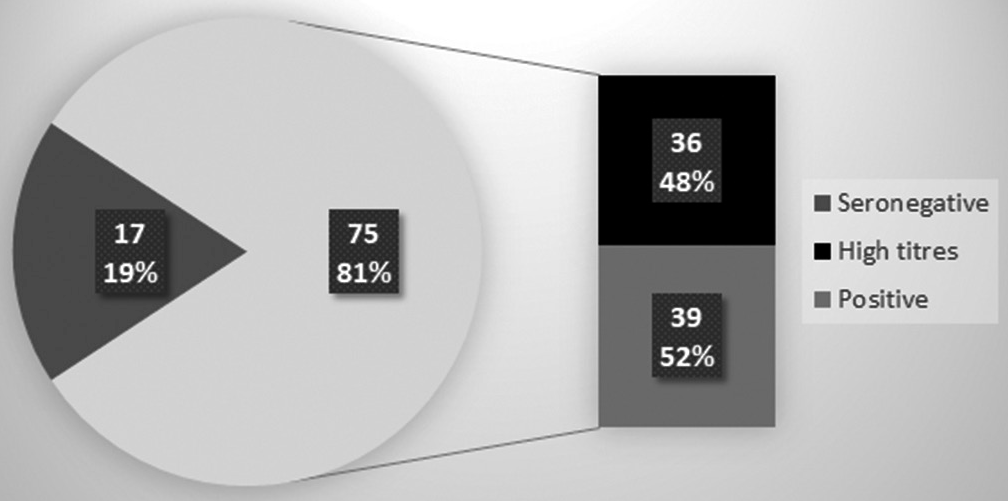
- Baseline seroprevalence of COVID-19 antibodies.
Patients with history of COVID-19 infection
Twelve of our patients had documented COVID-19 infections in the past (during the first wave of the pandemic in India), with 11 requiring hospitalization and 3 requiring oxygen support. They were managed according to the institutional protocols and guidelines issued during the first wave. All the 12 patients had detectable antibodies against the spike protein(S) of COVID-19 at the time of study entry, with nine patients (75%) having titers >250 U/ml. After two doses of the COVID-19 vaccine, all 12 of them had titers >250 U/ml.
Post-vaccination titers
During the study, 67 patients (69.8%) received two doses of Covaxin, while 29 (30.2%) received Covishield. Among the 17 patients who were seronegative at baseline, 4 (23.52%) were seropositive after the first dose of the vaccine and 11 (64.7%) were seropositive after the second dose, with high titers (“robust response”) being achieved in two patients (11.76%). No antibody response despite two doses of the vaccine was noted in six patients (35.29%) who were seronegative at baseline [Figure 2]. Because of the small number of patients who were seronegative at baseline (n = 17), factors associated with the development of an antibody response could not be studied.
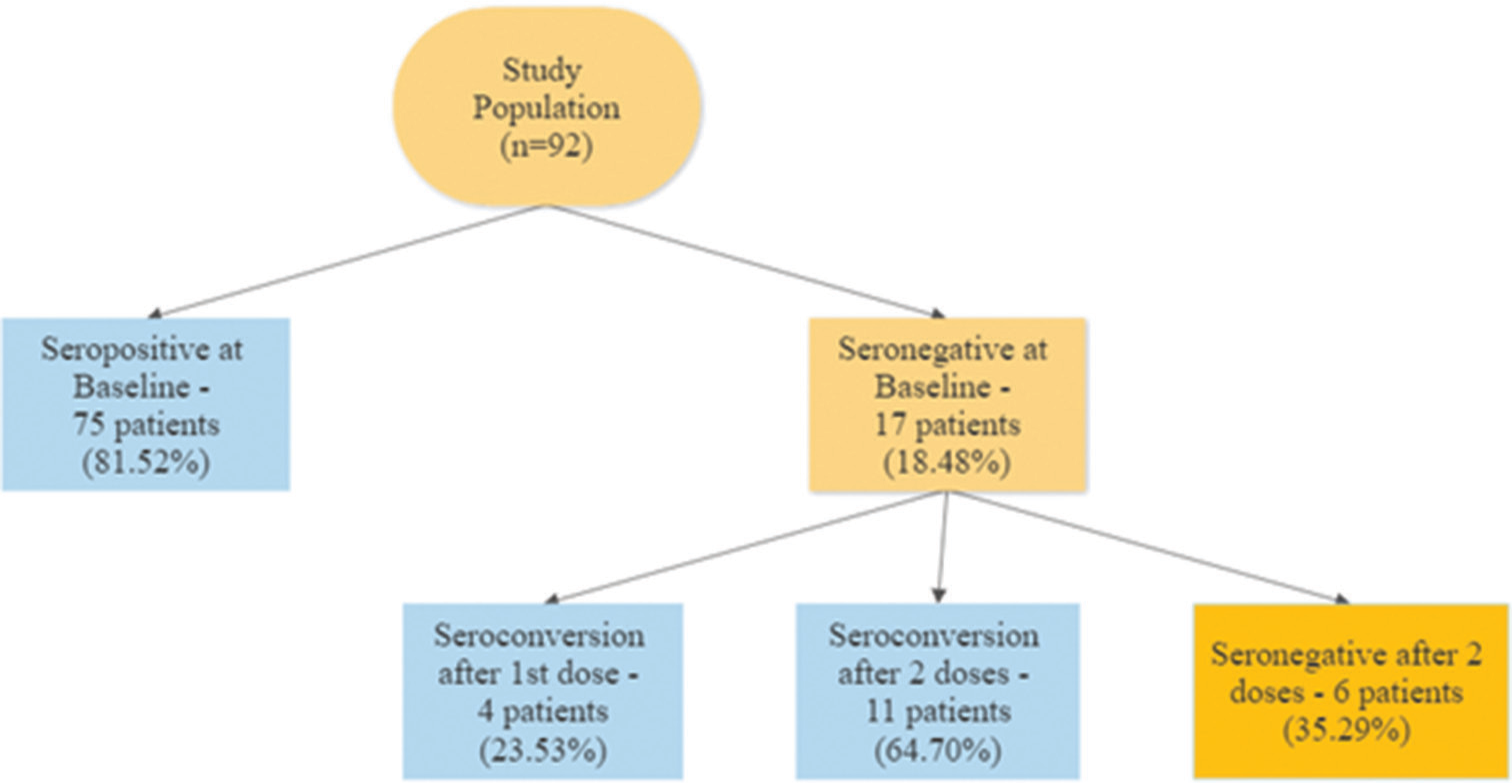
- Seroconversion rates at baseline and after the first and second doses of the vaccine.
Among those who were seropositive at baseline (n = 75), the proportion of patients with high titers (>250 U/ml) was 39% (n = 36) at baseline, 71% (n = 68) after the first dose and 81% (n = 78) after the second dose, thereby demonstrating a robust booster response to vaccination.
Adverse events following vaccination
Commonly reported adverse events included pain at injection site (23.96%) and fever lasting <48 hours (14.58%). No major adverse events such as anaphylaxis/hypersensitivity reactions were noted. Rates of adverse events were similar across both vaccine groups (Covaxin [33.14%], Covishield [28.26%], P = 0.61).
Three patients developed COVID-19 infection despite having received two doses of the vaccine, with the onset of symptoms occurring two days, three weeks, and two months, respectively, after the second dose. One of these three patients required temporary respiratory support in the form of supplemental oxygen, but all three recovered completely.
Discussion
The Oxford University developed the first candidate vaccine against COVID-19 - ChAdOx1 nCoV-19 (AZD1222), consisting of the replication-deficient simian adenovirus vector ChAdOx1. In November 2020, Phase III trials showed its efficacy in preventing COVID-19 infection and that it offered a high level of protection.6 After technology transfer from the Oxford University to the Serum Institute of India Pvt. Ltd., the same vaccine, now called SII-ChAdOx1 nCoV-19 (CovishieldTM) was produced in large quantities in India.
COVAXIN™ (BBV152) - Bharat Biotech developed India’s first indigenous, whole-virion, inactivated vaccine in collaboration with the Indian Medical Research Council and National Institute of Virology, Pune. Phase 3 trials concluded that BBV152 was immunogenic and highly efficacious against symptomatic and asymptomatic COVID-19 variant associated disease, particularly against severe disease in adults.7 In November 2021, the Technical Advisory Group for Emergency Use Listing listed COVAXIN against COVID-19 for emergency use. By the end of 2021, more than 1 billion vaccination doses had been administered in India.
Although this vaccination drive was considered the fastest vaccine roll-out in history, there was a dearth of studies and clinical trials in patients with ESKD. Patients with ESKD on maintenance hemodialysis who contract COVID-19 infection have higher mortality and morbidity rates than the general population.8-10 The inability of this population to mount an adequate immune response11 was a major concern.
India experienced a surge in the number of cases as well as mortality during the months of March and April 2021 – referred to as the “second wave”. Our study was performed during the latter part of this second wave in India. Interestingly, 75 patients (81%) in the study already had baseline seropositivity at study entry. Although adequate measures such as frequent testing and isolation, universal infection control precautions and prioritization of vaccination for the dialysis staff were taken, owing to the rampant community spread there still remained a high likelihood of undetected subclinical or mild infections during the first wave. Specific risk factors included frequent hospital visits, high numbers of close contacts before, during and after every dialysis session, and the impaired immune status of patients on hemodialysis.
Among the patients who were initially seronegative, good antibody responses (64.70% after two doses) were documented after vaccination, despite previous concerns of poor vaccine efficacy in this patient population. In fact, high titres were mounted in 11.76% of patients. This is similar to existing literature on the mRNA vaccine. A systematic review of 22 studies including patients on maintenance hemodialysis has documented seroconversion rates of 18% to 53% after 1 dose and in 70% to 96% after 2 doses of the vaccine.12
In our study, owing to the high seroprevalence at baseline, no significant analysis could be performed between patient-related factors and the degree of antibody response. However, other studies suggest that factors such as age, use of immunosuppressive drugs, low lymphocyte count, low serum albumin and longer dialysis vintage are independent predictors of a poor serological response.13-15
One of the major hurdles faced was the issue of vaccine hesitancy. However, after open discussions to address any potential concerns, 85% of our maintenance hemodialysis population came forward to be vaccinated. Motivation by peers was a major factor that helped accelerate this vaccination drive.
One of the limitations of the study was the high baseline seroprevalence, resulting in a much smaller number of patients in whom vaccine efficacy could be studied. However, high rates of seroprevalence, around 36.2%, has also been noted in another study among 356 patients on maintenance hemodialysis.16 Secondly, only the antibody response to vaccination was studied; the cellular component of the immune response was not assessed. However, recent evidence has validated antibody response as a correlate of protection from symptomatic infection.17 Thirdly, our study was limited to the effects of Covaxin and Covishield, and other vaccines could not be studied. Finally, although the initial response to vaccination has been adequate, concerns still exist about the durability of this immune response as some studies have shown that this sero-response in hemodialysis patients wanes over a period of 3-6 months.18,19
Conclusion
At the time when the study was conducted, 81% of patients with ESKD who were on maintenance hemodialysis were already seropositive for antibodies to COVID-19, indicating high rates of subclinical infection during the first wave. Among those who were seronegative at baseline, the seroconversion rate after two doses of Covaxin or Covishield was 64.70%.
Acknowledgement
We would like to thank Dr. K. Ramadevi, Professor and Director, Institute of Biochemistry, Madras Medical College. for providing logistic support in carrying out this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530-9.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973-83.
- [Google Scholar]
- What do we know about India's Covaxin vaccine? BMJ. 2021;373:n997. doi: 10.1136/bmj.n997
- [CrossRef] [PubMed] [Google Scholar]
- Applications of nanotechnology in vaccine development for coronavirus (SARS-CoV-2) disease (Covid-19) Int J Res Sci Inno. 2021;8:191-8.
- [CrossRef] [Google Scholar]
- 2021 Elecsys Anti-SARS-CoV-2 S, instructions for use (0928,9275190, V3.0, Dec 2021) Accessed 01 May 2023. https://www.fda.gov/media/144037/download
- [Google Scholar]
- Safety and efficacy of the ChAdOx1 NCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111.
- [Google Scholar]
- Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): Interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398:2173-84.
- [CrossRef] [Google Scholar]
- A clinical study on the changing dynamics of disease severity, management strategies and outcomes of COVID-19 in patients requiring haemodialysis. J Nephrol. 2021;34:999-1006.
- [CrossRef] [PubMed] [Google Scholar]
- Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540-8.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 in patients undergoing long-term dialysis in Ontario. CMAJ. 2021;193:E278-84.
- [CrossRef] [PubMed] [Google Scholar]
- Immune dysfunction in uremia 2020. Toxins. 2020;12:439. doi: 10.3390/toxins12070439
- [CrossRef] [PubMed] [Google Scholar]
- Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep. 2021;6:2292-304.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors and dynamics of the humoral and cellular immune response to SARS-CoV-2 mRNA vaccines in hemodialysis patients: A multicenter observational study. J Am Soc Nephrol. 2021;32:3208-20.
- [CrossRef] [PubMed] [Google Scholar]
- Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am J Kidney Dis Off J Natl Kidney Found. 2021;78:571-81.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody response to COVID-19 vaccination in patients receiving dialysis. J Am Soc Nephrol JASN. 2021;32:2435-38.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol. 2020;31:1969-75.
- [CrossRef] [PubMed] [Google Scholar]
- A Covid-19 milestone attained-A correlate of protection for vaccines. N Engl J Med. 2022;387:2203-6.
- [CrossRef] [PubMed] [Google Scholar]
- Waning humoral response 6 months after SARS-COV-2 vaccination with mRNA BNT162b2 vaccine in hemodialysis patients: Time for a boost. Kidney Int. 2021;100:1334-5.
- [CrossRef] [PubMed] [Google Scholar]
- Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583
- [CrossRef] [PubMed] [Google Scholar]







