Translate this page into:
Silent Hearing Loss in Kidney Transplant Patients Receiving Tacrolimus: A Fact or a Myth?
Corresponding author: Eman Nagy, Mansoura Nephrology and Dialysis Unit, Department of Internal Medicine, Mansoura University, Mansoura, Egypt. E-mail: emannagy@mans.edu.eg
-
Received: ,
Accepted: ,
How to cite this article: Abdulgalil AE, Elnagdy OH, Elnagdy NH, Nagy E. Silent Hearing Loss in Kidney Transplant Patients Receiving Tacrolimus: A Fact or a Myth? Indian J Nephrol. 2025;35:64-9. doi: 10.25259/ijn_503_23
Abstract
Background:
It has been claimed that tacrolimus may have harmful effects on the auditory system, where it has been linked to ototoxicity and sensorineural hearing loss (SNHL). We evaluated silent SNHL in kidney transplant recipients (KTRs) receiving tacrolimus and the different factors affecting it compared to healthy controls.
Materials and Methods:
In this case control study, hearing functions were studied in 42 KTRs receiving tacrolimus as maintenance immunosuppressive therapy for more than 3 months in comparison to 27 age- and gender-matched healthy subjects using tympanometry, pure-tone audiometry (PTA), extended high frequency audiometry (EHFA), and transient evoked oto-acoustic emissions (TEOAEs). Also, different factors were studied in relation to SNHL.
Results:
PTA showed that 23.8%, 21.4%, and 4.8% had mild, moderate, and severe SNHL, respectively. One-fifth of KTRs had severe SNHL, according to EHFA. According to TEOAEs, 28.6% of KTRs had abnormal hearing. There was a significant positive correlation between the tacrolimus trough levels and the results of both the PTA (P = 0.002) and EHFA (P = 0.035) tests.
Conclusion:
SNHL was detected in about half of the studied KTRs. Silent SNHL in KTRs might be associated with higher tacrolimus trough levels.
Keywords
Hearing loss
Renal transplant
Tacrolimus
Immunosuppression
Ototoxicity
Introduction
Kidney transplantation is the preferred treatment for patients with end-stage kidney disease (ESKD).1 The number of transplants performed over the past three decades has continuously increased. The Egyptian experience exceeded 20,000 transplants up to the year 2020. The 10-year graft and patient survival rates in Mansoura Urology and Nephrology Center (one of the largest transplant centers in Egypt) were 65.5% and 77.8%, respectively.2
The mainstay of solid organ transplant immunosuppression is calcineurin inhibitors (CNIs). Tacrolimus remains the first-line treatment option, and this mandates an updated assessment of its safety profile. It has a narrow therapeutic window and might result in different adverse events such as nephrotoxicity, neurotoxicity, and new-onset diabetes. These adverse events are mostly related to either the long-term usage of tacrolimus or its increased trough level.3
It has been claimed that tacrolimus has harmful effects on the auditory system in some studies,4-7 where it has been linked to ototoxicity and sensorineural hearing loss (SNHL). The cause of SNHL is usually the combined result of many factors. These neurotoxic side effects could be involved in the development of hearing impairment after transplant.4 This work aimed to study silent hearing loss in transplant recipients (KTRs) receiving tacrolimus and the different factors affecting it in comparison to age- and gender-matched healthy controls.
Materials and Methods
This case control study was conducted between May 2022 and November 2022 in the Mansoura University Nephrology Unit and Audiology Department, Faculty of Medicine, Mansoura University, Egypt. The study was approved by the Institutional Review Board. An informed written consent was obtained from all patients included in the study or their caring relatives prior to the study.
Forty-two KTRs, aged between 18 and 60 years and treated with tacrolimus continuously as maintenance immunosuppressive therapy for more than 3 months and attending the kidney transplant follow-up clinic during the duration of the study, were included in the transplant group. All KTRs included in this study had grafts from living, related donors. Most KTRs were maintained on prednisolone, mycophenolate mofetil (MMF), and tacrolimus. The doses of these drugs after one year of kidney transplantation as adopted by our center are as follows: prednisolone 5 mg/day. MMF 1500 mg/day and tacrolimus dose that kept its trough level between 5 and 6 ng/mL. The control group included 27 healthy subjects who were matched for age and gender with the transplant patients.
Complicated diabetic cases, patients with any other middle ear disease as otitis media or perforated drums in otoscopic examination, have used other ototoxic drugs within the last 3 months (antibiotics as aminoglycoside, erythromycin, vancomycin, or furosemide, etc.), had a history of hereditary or acquired hearing loss problems due to other reason (genetic syndromes with hearing loss as Alport, acoustic trauma, those with recurrent upper respiratory tract infection, neurological psychiatric problems, Meniere’s disease, have intracranial pathology that may cause hearing loss, malignancy and have been receiving chemotherapy, have had ear trauma or surgery were excluded from the study.
Group sample sizes of 41 in group 1 (transplant group) and 21 in group 2 (control group) achieve 85.186% power to detect a difference between the group proportions of −0.3500. The proportion in group 1 (the transplant group) is assumed to be 0.5000 under the null hypothesis and 0.1500 under the alternative hypothesis. The proportion in group 2 (the control group) is 0.5000. The test statistic used is the two-sided Z-Test with unpooled variance. The significance level of the test is 0.0700.
All patients in both the groups were subjected to detailed clinical examination, including history of subjective hearing loss, purulent discharge from ear, tinnitus, or ototoxic drug exposure); investigations (including complete blood count (CBC), serum albumin, creatinine, magnesium, fasting blood sugar, and tacrolimus trough level [FK level]). Tacrolimus level was used as an average reading over the last 3 months. In addition, tympanometry, pure-tone audiometry (PTA), extended high frequency audiometry (EHFA), and transient evoked oto-acoustic emissions (TEOAEs) were done for all patients.
PTA was performed in a sound-treated room to minimize background noise according to the law of the European Economic Community. Madsen Itera 2 Clinical Audiometer (Natus, Denmark) was used to conduct the PTA examination. Thresholds of air conduction were estimated for 250–8000 Hertz (Hz).
EHFA was performed in a sound-proof room using Interacoustics AC 40, (Interacoustic, Assens, Denmark) at 10,000, 12,000, 16,000 and 20,000 Hz. Tympanometry was carried out by interacoustics AT 235 impedance audiometer (Interacoustic, Assens, Denmark). Type A tympanogram was found in all cases.
Acoustic reflex (AR): interacoustics AT 235 impedance audiometer (Interacoustic, Assens, Denmark) was used to measure ipsilateral AR at 500, 1000, 2000 and 4000 Hz. Scout (Bio-logic, United Kingdom) TEOAES was utilized to conduct oto-acoustic emissions (OAEs). Normal hair cell function was indicated by PASS indicated while abnormal hair cell function with an enhanced risk of hearing loss in the future was indicated by REFER.
The collected data were analyzed using SPSS 25 for personal computers. The Shapiro–Wilk test was used to test the normality of numeric variables. For parametric and non-parametric variables, quantitative data was expressed as mean±SD, or median (minimum-maximum), while qualitative data was expressed as number and percentages. Independent samples t-test was used to compare parametric variables, while the Mann-Whitney U test was used to compare non-parametric variables between two groups. The Chi-square test was used to compare qualitative variables with each other. Spearman correlation was used to correlate different variables with audiometric data. P values less than 0.05 were considered to be significant.
Results
The current study included 42 KTRs with a mean age of 36 years. The majority of them were males (71.4%) and hypertensives (64.3%). The median duration of the transplant was 43 months. All the patients were maintained on prednisolone, tacrolimus, and mycophenolate mofetil. The median serum creatinine of the patients was 1.2 mg/dL, while the mean serum magnesium level was 1.66 mg/dL. The median FK level was 5.89 ng/mL. Twenty-seven controls, matched for age and gender with KTRs, were studied [Table 1].
| Transplant patients (n = 42) | Control group (n = 27) | P value | |
|---|---|---|---|
| Age, years |
36.14 ± 10.53 33.5 (20-58) |
35.22 ± 8.50 | 0.704* |
| Gender | 0.461*** | ||
| Male | 30 (71.4%) | 17 (63%) | |
| Female | 12 (28.6%) | 10 (37%) | |
| Original kidney disease | No | ||
| Hypertension | 4 (9.5%) | ||
| Diabetes | 1 (2.4%) | ||
| Glomerulonephritis | 8 (19%) | ||
| Pregnancy related | 7 (16.7%) | ||
| Autosomal dominant polycystic kidney disease | 2 (4.8%) | ||
| Neurogenic bladder with reflux nephropathy | 2 (4.8%) | ||
| Stone kidney disease | 2 (4.8%) | ||
| Bilateral urinary tract obstruction | 1 (2.4%) | ||
| Unknown | 15 (35.7%) | ||
| Pre-transplantation dialysis | 35 (83.3%) | ||
| Duration of pre-transplantation dialysis (months) | 24 (1–216) | ||
| Duration of transplantation (months) | 43 (3–96) | ||
| Diabetes | 3 (7.1%) | No | |
| Duration of diabetes (years) | 12 (4–14) | ||
| Hypertension | 27 (64.3%) | No | |
| Duration of hypertension (years) | 10 (2–20) | ||
| Steroid dose (mg/day) | 5 (5–30) | ||
| Tacrolimus dose (mg/day) | 3.5 (1.75–8) | ||
| Serum creatinine (mg/dL) | 1.2 (0.7–5.4) | 0.8 (0.7–1.1) | <0.001** |
| White blood cells (109/L) | 8.25 (3.2–13.9) | 5.4 (4.4–7.5) | <0.001** |
| Neutrophil to lymphocyte ratio | 1.73 (0.96–10.32) | ||
| Hemoglobin (gm/dL) | 13.02 ± 2.31 | 13.22 ± 0.89 | 0.663* |
| Platelets (109/L) | 221.67 ± 66.05 | 251.85 ± 31.26 | 0.030* |
| Serum calcium (mg/dL) | 9.34 ± 0.87 | 9.39 ± 0.43 | 0.800* |
| Serum phosphorus (mg/dL) | 3.57 ± 1.21 | 4.19 ± 0.32 | 0.030* |
| Serum albumin (gm/dL) | 4.20 ± 0.52 | 4.5 ± 0.23 | 0.023* |
| Serum magnesium (mg/dL) | 1.66 ± 0.21 | 1.9 ± 0.08 | <0.001* |
| Tacrolimus trough level (ng/mL) | 5.89 (2–11) |
Data were expressed by N (%), mean ± SD, median (minimum-maximum). *P value was computed by independent samples t-test. **P value was computed by Mann-Whitney U test. ***P value was computed by Chi-square test. The bold values denote that these values are statistically significant.
Audiometry of KTRs showed that half of the patients had normal hearing according to PTA, while 23.8%, 21.4%, and 4.8% of them had mild, moderate, and severe SNHL, respectively. One-fifth of KTRs had severe SNHL when tested by EHFA. According to TEOAE, 28.6% had abnormal hair cell function, with an enhanced risk of hearing loss in the future [Table 2]. The comparison of different Hz between KTRs and the control group showed that there were statistically significant differences in hearing thresholds between them with regard to 250, 500, 4000, and 8000 Hz [Figure 1]. Mean FK level was significantly higher in KTRs with SNHL as diagnosed by PTA (P = 0.019) [Figure 2].
| Transplant patients (N = 42) | |
|---|---|
| PTA | |
| Normal | 21 (50%) |
| Mild | 10 (23.8%) |
| Moderate | 9 (21.4%) |
| Severe | 2 (4.8%) |
| EHFA | |
| Normal | 19 (45.2%) |
| Mild | 3 (7.1%) |
| Moderate | 8 (19%) |
| Moderate to severe | 3 (7.1%) |
| Severe | 9 (21.4%) |
| TEOAE | |
| Refer | 12 (28.6%) |
| Partially pass | 7 (16.7%) |
| Pass | 23 (54.8%) |
PTA: Pure-tone audiometry; EHFA: Extended high frequency audiometry; TEOAE: Transient evoked oto-acoustic emissions. The data were expressed as N (%).
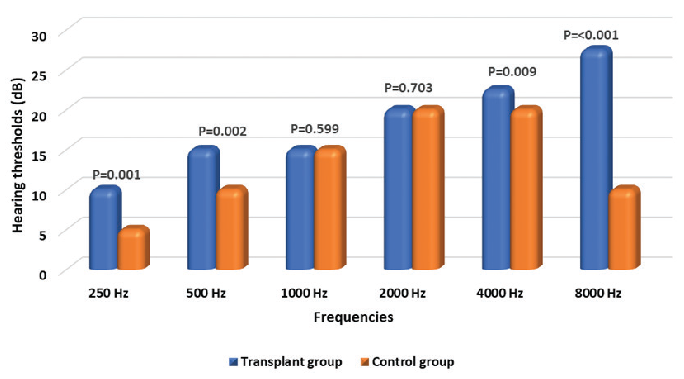
- Comparison of median hearing threshold in dB between transplant group and control as regards different HZ using pure-tone audiometry. P value was computed by Mann-Whitney U test.
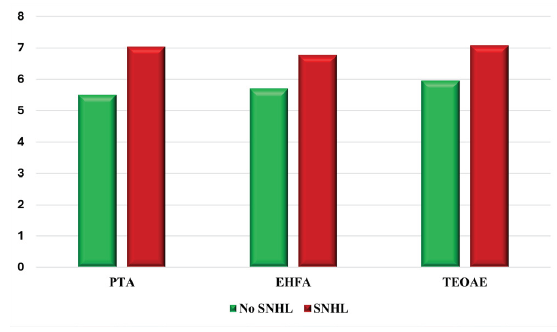
- Comparison between with and without sensorineural hearing loss as regard mean tacrolimus trough level. PTA: Pure-tone audiometry, EHFA: Extended high frequency audiometry, SNHL: sensorineural hearing loss, TEOAE: Transient evoked oto-acoustic emissions. P value was computed using independent sample student t-test.
There was a significant positive correlation between tacrolimus trough levels and the results of both the PTA (rho = 0.469, P = 0.002) and EHFA (rho = 0.326, P = 0.035) tests, while there was no significant correlation with the TEOAE results [Table 3]. In addition, Spearman correlation between the different Hz and different variables showed that tacrolimus level was positively correlated with 4000 and 8000 Hz (rho = 0.342 and 0.424, p = 0.027 and 0.005, respectively). However, there was no significant correlation between the serum magnesium and tacrolimus levels (rho = −0.096, P = 0.680) [Table 4, Figure 3].
| PTA | EHFA | TEOAE | ||
|---|---|---|---|---|
| Age | Rho | 0.080 | 0.207 | -0.168 |
| P value | 0.615 | 0.188 | 0.286 | |
| Duration of pre-transplantation dialysis | Rho | 0.003 | −0.185 | 0.077 |
| P value | 0.987 | 0.288 | 0.660 | |
| Duration of transplantation | Rho | −0.208 | −0.176 | 0.086 |
| P value | 0.186 | 0.264 | 0.586 | |
| Duration of hypertension | Rho | 0.144 | −0.042 | −0.228 |
| P value | 0.493 | 0.841 | 0.272 | |
| Serum creatinine | Rho | −0.161 | −0.210 | 0.145 |
| P value | 0.310 | 0.182 | 0.360 | |
| Serum calcium | Rho | 0.179 | −0.132 | −0.008 |
| P value | 0.426 | 0.559 | 0.971 | |
| Serum phosphorus | Rho | −0.098 | −0.173 | −0.034 |
| P value | 0.673 | 0.454 | 0.883 | |
| Serum magnesium | Rho | 0.015 | 0.021 | 0.129 |
| P value | 0.949 | 0.927 | 0.578 | |
| Tacrolimus trough level | Rho | 0.469 | 0.326 | −0.281 |
| P value | 0.002 | 0.035 | 0.072 | |
| Steroid dose | Rho | 0.023 | −0.203 | 0.051 |
| P value | 0.883 | 0.197 | 0.750 | |
| Tacrolimus dose | Rho | 0.262 | 0.253 | −0.129 |
| P value | 0.094 | 0.107 | 0.415 |
PTA: Pure-tone audiometry; EHFA: Extended high frequency audiometry; TEOAE: Transient evoked oto-acoustic emissions. The bold values denote that these values are statistically significant.
| Parameter | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | 8000 Hz | |
|---|---|---|---|---|---|---|---|
| Age | Rho | −0.015 | −0.091 | −0.136 | −0.032 | 0.136 | 0.109 |
| P value | 0.927 | 0.565 | 0.391 | 0.842 | 0.389 | 0.492 | |
| Duration of pre-transplantation dialysis | Rho | 0.082 | −0.046 | −0.168 | −0.029 | −0.178 | −0.066 |
| P value | 0.638 | 0.795 | 0.334 | 0.870 | 0.305 | 0.705 | |
| Duration of transplantation | Rho | −0.118 | −0.053 | −0.152 | −0.167 | −0.140 | −0.223 |
| P value | 0.456 | 0.740 | 0.338 | 0.290 | 0.376 | 0.155 | |
| Duration of hypertension | Rho | 0.199 | 0.133 | 0.079 | −0.089 | 0.145 | 0.082 |
| P value | 0.341 | 0.525 | 0.707 | 0.673 | 0.488 | 0.696 | |
| Serum creatinine | Rho | −0.257 | −0.207 | −0.141 | −0.159 | 0.011 | −0.046 |
| P value | 0.101 | 0.189 | 0.374 | 0.314 | 0.947 | 0.771 | |
| Serum calcium | Rho | 0.093 | −0.091 | 0.090 | −0.156 | 0.271 | 0.238 |
| P value | 0.680 | 0.687 | 0.690 | 0.488 | 0.223 | 0.287 | |
| Serum phosphorus | Rho | −0.126 | −0.103 | 0.211 | 0.055 | 0.221 | 0.096 |
| P value | 0.586 | 0.658 | 0.358 | 0.814 | 0.336 | 0.679 | |
| Serum magnesium | Rho | 0.127 | 0.153 | 0.194 | 0.273 | −0.037 | 0.068 |
| P value | 0.583 | 0.507 | 0.400 | 0.231 | 0.873 | 0.768 | |
| Tacrolimus trough level | Rho | 0.081 | 0.109 | 0.021 | −0.001 | 0.342 | 0.424 |
| P value | 0.609 | 0.491 | 0.893 | 0.995 | 0.027 | 0.005 | |
| Steroid dose | Rho | −0.200 | −0.150 | −0.021 | −0.145 | −0.047 | −0.045 |
| P value | 0.203 | 0.343 | 0.894 | 0.361 | 0.769 | 0.779 | |
| Tacrolimus dose | Rho | 0.173 | 0.099 | 0.010 | 0.110 | 0.128 | 0.199 |
| P value | 0.272 | 0.531 | 0.948 | 0.490 | 0.419 | 0.206 |
The bold values denote that these values are statistically significant.
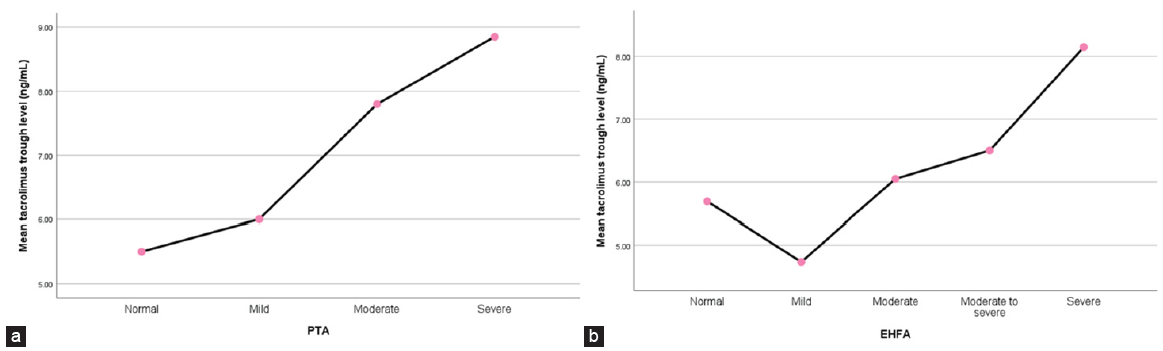
- (a) Summary point plot mean of tacrolimus trough level by PTA and (b) summary point plot mean of tacrolimus trough level by EHFA. PTA: Pure-tone audiometry, EHFA: Extended high frequency audiometry.
Discussion
Hearing impairment is a disorder that may affect KTRs’ quality of life. Numerous adverse events of CNIs are well known, though few studies on their effect on hearing have been published. The results of the current study showed that half of KTRs had non-subjective SNHL according to the findings of the PTA test, where 21% and 4% had moderate and severe SNHL, respectively. These findings are consistent with those of Rifai et al.,5 who investigated hearing disorders in 695 liver transplant patients receiving immunosuppressive drugs using a questionnaire. They discovered that 141 patients had hearing loss and/or tinnitus, with a significant positive correlation to tacrolimus in uni- and multi-variate analyses, where more than a third of the patients with hearing loss were on tacrolimus. Also, these results cope with the findings of Rifai et al.,6 who studied 70 liver transplant cases (56% of them were on cyclosporine and 34% were on tacrolimus) using PTA and found that 50% of 18 patients without subjective hearing loss had moderate or severe SNHL, while those with subjective hearing loss had a significantly worse outcome as tested by PTA. Such results were consistent also with those of Fortes et al.,7 who discovered profound hearing impairments in 24 liver transplant recipients given tacrolimus, when compared to those receiving cyclosporine by PTA.
On performing the EHFA test, 19%, 7%, and 21% of the KTRs in our study had moderate, moderate to severe, and severe SNHL, respectively. These findings are in agreement with the results of a recent study by Simsir et al.,8 who evaluated hearing defects in 46 KTRs (30 of them were on tacrolimus) and found that a high percentage of their patients had hearing impairment, and this percentage increased as the frequency of sound increased, reaching up to 76% of their transplant patients.
The TEOAE results of KTRs in this study showed that nearly 29% of them had hearing abnormalities with an enhanced future risk of hearing loss, although these patients were asymptomatic at the time of the study. These findings agree with those reported by Fadel et al.,9 who studied 40 pediatric transplant cases receiving tacrolimus and found that 25% of the cases had hearing impairment. They also reported that children with longer pre-transplant duration on dialysis or biopsy proven rejection had significantly higher hearing affection than those without. However, in our study, there was no correlation between the duration of pre-transplant dialysis and the results of the different audiometric tests used in the study.
Different factors were studied in correlation to the results of different audiometric tests in this study. The tacrolimus trough level was the only factor found to have a significant positive correlation with the results of both the PTA and EHFA tests. All factors did not have a significant correlation with the results of the TEOAE test. These results are in agreement with other studies and case reports that showed that a high tacrolimus level was associated with SNHL.10-12 However, other studies could not find a correlation between tacrolimus blood levels and SNHL.8,9
A recent case report12 presented the case of a 51-year-old KTR who developed sudden vestibular disorders and SNHL after receiving tacrolimus; this condition was associated with severe hypomagnesemia. The patient’s clinical condition improved after the correction of hypomagnesemia. The authors claimed that this condition was due to tacrolimus-induced hypomagnesemia. We studied the correlation of different factors, including magnesium, to SNHL and found that, although the mean serum magnesium level was below normal in the studied cases, it was not significantly correlated with either the results of different audiometric tests done for the patients or the tacrolimus level. This indicates that a low serum magnesium level is not the cause of SNHL related to tacrolimus. To our knowledge, we did not find any other studies that investigated the correlation of serum magnesium level with SNHL in KTRs receiving tacrolimus.
SNHL and other hearing disorders may be associated with the use of immunosuppressive drugs such as tacrolimus. A dose-dependent mechanism, like neurotoxicity, has been suggested to be the cause.13 Different mechanisms have been suggested, including microscopic thromboembolic events, reduced molecular diffusion across the blood–inner ear barrier, and modification of the P-glycoprotein multidrug extrusion pump of the inner ear plasma membrane. The latter mechanism appears to cause the accumulation of considerable amounts of ototoxic substances within the inner ear.14 Sudden SNHL was reported in several patients exposed to high blood levels of tacrolimus. There is evidence that vasculopathy and endothelial dysfunction, which disturb the blood-brain barrier, are the causes of CNI-related neurotoxicity. The vascular injury to the inner ear’s capillary endothelial cells, causing disturbance of the blood-inner ear barrier, was believed to contribute to hearing loss.15
The clinical care for KTRs should include periodic audiometric evaluation for early detection of silent SNHL, which could be reversible by minimizing the dose of tacrolimus to the least effective dose to prevent graft loss and protect hearing. The impact of hearing loss on a KTR’s quality of life should be kept in mind. Awareness of this potential of adverse event of tacrolimus may help with early detection and treatment.
SNHL was detected in about half of the studied KTRs. Silent SNHL might be associated with higher tacrolimus trough levels.
Conflicts of interest
There are no conflicts of interest.
References
- Epidemiology of chronic kidney disease in children: A report from Lithuania. Medicina. 2021;57:112.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- History of renal transplantation in the Arab world. Arch Hellen Med. 2020;37:208-13.
- [Google Scholar]
- An update on the safety of tacrolimus in kidney transplant recipients, with a focus on tacrolimus minimization. Expert opinion on drug safety. 2019;18:285-94.
- [CrossRef] [PubMed] [Google Scholar]
- Hearing status in pediatric renal transplant recipients. Exp Clin Transplant. 2015;13:324-8.
- [CrossRef] [PubMed] [Google Scholar]
- A new side effect of immunosuppression: High incidence of hearing impairment after liver transplantation. Liver Transplantation. 2006;12:411-5.
- [CrossRef] [PubMed] [Google Scholar]
- High rate of unperceived hearing loss in patients after liver transplantation. Clin Transplant. 2012;26:577-80.
- [CrossRef] [PubMed] [Google Scholar]
- Audiometric changes in patients undergoing liver transplantation using distinct immunosuppressive protocols. Liver Transpl. 2008;14:509-11.
- [CrossRef] [PubMed] [Google Scholar]
- Hearing impairments as an overlooked condition in kidney transplant recipients. Transplant International 2022:87.
- [Google Scholar]
- Hearing assessment in egyptian children with chronic renal failure on regular hemodialysis and renal transplantation children. Therapeutic Apheresis and Dialysis. 2022;26:960-8.
- [CrossRef] [PubMed] [Google Scholar]
- Sudden hearing loss associated with tacrolimus after pediatric renal transplant. Exp Clin Transplant. 2013;11:562-4.
- [CrossRef] [PubMed] [Google Scholar]
- Sensorineural deafness following tacrolimus use. experimental and clinical transplantation. Official Journal of the Middle East Society for Organ Transplantation. 2018;18:110-1.
- [Google Scholar]
- Vestibular disorders after kidney transplantation: Focus on the pathophysiological mechanisms underlying the vertical nystagmus associated with tacrolimus-related hypomagnesamia. Int J Environ Res Public Health. 2022;19:2260.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Severe hearing loss after liver transplantation. Transplant Proc. 2005;37:1918-19.
- [CrossRef] [PubMed] [Google Scholar]
- Cyclosporin a inhibits the extrusion pump function of p-glycoprotein in the inner ear of mice treated with vinblastine and doxorubicin. Brain Res. 2001;901:265-70.
- [CrossRef] [PubMed] [Google Scholar]
- Disruption of mdr1a p-glycoprotein gene results in dysfunction of blood-inner ear barrier in mice. Brain Res. 2000;852:116-26.
- [CrossRef] [PubMed] [Google Scholar]








