Translate this page into:
Skin Colonizers and Catheter Associated Blood Stream Infections in Incident Indian Dialysis Patients
Address for correspondence: Prof. Anna T. Valson, Department of Nephrology, Christian Medical College Vellore, Ida Scudder Road, Vellore, Tamil Nadu – 632004, India. E-mail: annavalson@cmcvellore.ac.in
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Skin colonization is a risk factor for multi-drug resistant (MDR) catheter-associated bloodstream infections (CABSI). This study aimed to determine the prevalence and spectrum of skin colonizing MDR organisms in incident HD patients and their correlation with CABSI.
Methods:
This single-center prospective cohort study included consecutive adult incident HD patients who underwent tunneled or non-tunneled internal jugular vein HD catheter insertion between June 1, 2017 and October 31, 2017. Nasal, axillary, and exit site swabs were obtained prior to catheter insertion, at 14–21 days, and 28–35 days after catheter insertion.
Results:
Forty-three patients (69.7% male, 32.5% diabetic) were included and provided baseline swabs, while 29 and 10 patients respectively were available for follow-up swabs. MDR bacterial colonization, MRSA colonization, and MDR gram-negative colonization on the baseline set of swabs were seen in 76.7%, 69.7%, and 9.3% patients respectively. Of the 29 patients with at least two consecutive sets of swabs, 79.3% showed persistent colonization by MDR gram-positive organisms, most commonly by MRSA. Six patients developed a CABSI during the follow-up period (incidence rate 3.7 per 1000 patient days), 83.4% were gram negative, and in only one instance (16.6%) was the bacterial strain identical to that which had previously colonized the skin.
Conclusions:
Three-fourths of HD patients were colonized by MDR bacteria prior to HD initiation. Despite the majority being persistently colonized by MDR gram-positive organisms, CABSIs were predominantly gram negative.
Keywords
Catheter-related infections/microbiology
hemodialysis
India
nasal mucosa/microbiology
skin/microbiology
Introduction
The overwhelming majority of patients initiating hemodialysis (HD) in India do so via non-tunneled or tunneled dialysis catheters[123] because of delayed referral to a nephrologist.[4] The use of catheters is associated with an increased risk for fatal infections, all-cause and cardiovascular mortality.[5] Not surprisingly, catheter-associated bloodstream infections (CABSIs) are the most common cause of sepsis in this group.[6]
We have previously shown that the microbiological spectrum of CABSIs in India differs from that in other countries, in that CABSIs occur early, gram-negative infections predominate, and a large proportion are multi-drug resistant (MDR).[7] Since colonization of the skin and nasal mucosa is an important risk factor for CABSIs, we wondered if the propensity to develop gram-negative CABSIs in the Indian subcontinent was due to colonization with gram-negative bacteria either prior to or after the initiation of dialysis. This study therefore aimed to determine the prevalence and spectrum of skin colonizing MDR organisms in incident HD patients at baseline, 2 and 4 weeks after dialysis catheter insertion and determine whether there was any correlation between colonizing organisms and organisms causing CABSI in this cohort.
Materials and Methods
In this prospective observational cohort study, after obtaining written, informed consent, we included consecutive adult (≥18 years) outpatient incident HD patients who underwent tunneled or non-tunneled internal jugular vein HD catheter insertion (Mahurkar™, Covidien AG, Mansfield, MA) at our dialysis unit between June 1, 2017 and October 31, 2017 and who were likely to continue dialysis for ≥ 2 weeks at our center. We excluded patients who had HD catheters inserted at other centers, femoral HD catheter insertion, central venous catheters inserted for purposes other than HD (plasmapheresis, vasopressor or antibiotic administration, etc.), HD catheters inserted in ICUs, patients who had received HD or peritoneal dialysis in the past, patients unlikely to continue dialysis for ≥2 weeks at our center after HD catheter insertion. Data were prospectively collected in a standardized proforma and laboratory data was obtained from the hospital information system. All patients were followed up at the dialysis unit till the first episode of CABSI or their last dialysis session at our center with the incident dialysis catheter. The study was approved by the Institutional Review Board and Ethics Committee of our institution. All procedures performed were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The aims and objectives of this study were as follows:
Primary objective: To determine the prevalence of colonization of nasal mucosa, axillary skin and proposed catheter exit site by MDR organisms in adult incident HD patients prior to HD initiation
Secondary objectives:
-
To determine the factors associated with colonization by MDR organisms prior to HD initiation
-
To determine the change (if any) in colonization pattern between the baseline set of swabs and swabs obtained ≥2 weeks (14–21 days) and ≥4 weeks (28–35 days) after HD initiation
-
To determine the proportion of patients who show persistent colonization with MDR organisms
-
To determine the correlation (if any) between skin colonizing organisms and organisms causing CABSI in this cohort
Protocol for catheter insertion and manipulation
HD catheters were inserted by nephrology residents under ultrasound guidance in a dedicated surgical suite within the dialysis facility, either independently or under consultant supervision, using the modified Seldinger technique, with the right internal jugular vein being the preferred site for insertion. Standard aseptic precautions were followed for catheter insertion using skin disinfection with 2% chlorhexidine gluconate. After catheter insertion, mupirocin ointment was applied at the exit site, which was then covered with sterile gauze and transparent dressing (Tegaderm™, 3M™, St. Paul, MN).
Before, during, and after an HD session, catheter manipulation by dialysis staff was carried out under sterile barrier precautions. The catheter exit site and port caps were cleansed with 2% chlorhexidine gluconate, caps were removed, hubs were cleansed with 2% chlorhexidine gluconate, a syringe was attached to aspirate heparin lock solution, lumens were flushed with saline, following which dialysis tubing was connected. Catheter hubs were kept soaked in chlorhexidine solution during the HD sessions. At the end of HD, fresh sterile gauze and a transparent dressing were applied at the exit site. Mupirocin ointment was applied at the exit site only for the first 1 week after catheter insertion. The catheter lumen was locked with heparin solution (1000 units/ml) as per the internal volume specified by the manufacturer. No antibiotic lock solutions were used.
Patients underwent HD for 4 h, twice or thrice weekly, with a low flux polysulfone dialyzer (FX8, Fresenius Medical Care AG & Co., Bad Homburg, Germany). Dialyzers were not reused. Water purity was monitored once a month to ensure microbial contamination <200 CFU/ml and endotoxin levels <0.2 EU/ml.
Protocol for swab collection
Swabs were obtained by a single investigator (VA) from four body sites (left and right anterior nares – 1 swab, right and left axilla – 1 swab each, proposed catheter exit site in the neck – 1 swab) prior to HD catheter insertion. All swabs were pre-moistened with sterile normal saline. Nasal swabs were obtained using a standard protocol as described previously.[8] In brief, the swab was inserted into the nasal vestibule (approximately 2 cm) and rotated against the mucosa for 5 seconds while applying gentle pressure so as to lightly bend the swab. Using the same swab, the procedure was repeated for the other nostril. HD catheter exit-site swabs and axillary swabs were obtained by rolling a pre-moistened swab once, forwards and backward at the proposed exit site and bilateral axillae. Swabs were obtained from the same sites at 14-21 days (first follow-up swab) and 28-35 days (second follow-up swab) after HD catheter insertion, prior to a HD session. Swabs from the catheter exit site were obtained prior to skin cleansing of the exit site with an antiseptic solution.
The protocols for HD catheter insertion and manipulation as well as the dialysis protocol are given in the Supplementary Material.
Laboratory testing methods
After collection, all swabs were placed in the corresponding transport tube and subjected to microbiological analysis within one hour. Before inoculation onto blood agar plates, swabs were pre-incubated with nutrient broth at 37°C for 2 h. Colonies were isolated by inoculating 100 μL aliquots onto Columbia agar supplemented with 5% sheep blood. Agar plates were subsequently incubated at 37°C under ambient atmosphere for 48 h. After incubation, significant growth was identified as colonies and was characterized by using standard microbiological methods.[9] Antimicrobial susceptibility testing was performed by using the standard Kirby-Bauer disc diffusion method (HiMedia Laboratories Pvt. Ltd., Mumbai, India) as per Clinical and Laboratory Standards Institute (CLSI) recommendations. In patients who developed a CABSI due to an organism that had previously been cultured on a skin swab, or whose exit site swab grew an organism that had previously been cultured at another site, molecular characterization was done using multi-locus sequence typing (MLST) or multiplex PCR, to determine if the two strains were similar.
Protocol for diagnosis of CABSI
In all patients with clinical features suggestive of CABSI (see below), paired blood cultures (10 ml each) were obtained from the peripheral blood and venous catheter hub under sterile precautions and inoculated in culture media (BACT/ALERT® SA, bioMérieux Inc, Durham, NC). Peripheral blood cultures were obtained from the median cubital vein or the dialysis circuit.
Definitions
Drug resistance patterns were defined as per the International Expert Proposal for Interim Standard Definitions for Acquired Resistance.[10] CABSI was defined as per the Center for Disease Control and Prevention (CDC) guidelines[11] with a diagnosis of coagulase negative staphylococcus (CoNS) CABSI being made only if both catheter and peripheral blood cultures grew the same organism, while one positive blood culture (catheter hub or peripheral blood or both) was sufficient for other organisms. Persistent Colonizers were defined as those who grew the same drug resistant organism on two successive swabs from the same site. Persistent colonizers were further classified as persistent intrinsic colonizers (those in whom the organism was identified on the baseline and consecutive follow-up swab, indicating that persistent colonization existed prior to dialysis initiation), and persistent acquired colonizers (where the organism was not identified on the baseline swab, but grew in both follow-up swabs, suggesting that the organism was acquired after initiation on dialysis). All others were termed transient colonizers.
Statistical analysis
Categorical variables were summarized using counts and percentages. Quantitative variables were summarized using mean and standard deviation or median and IQR. Chi-square test was used to compare the proportions between categorical variables. The Independent t-test was used to compare the means between two groups for normally distributed continuous variables and the Mann–Whitney U test was used for skewed variables. Changes in proportions were compared using the N-1 Chi-square test (available at https://www.medcalc.org/calc/comparison_ of_proportions.php). A 5% level of significance was considered significant. Statistical analysis was carried out using STATA v. 13.1 (StataCorp. College Station, TX)
Results
Figure 1 gives the STROBE diagram for the study. A total of 494 patients underwent catheter insertion during the study period of which 451 were excluded for various reasons. Forty-three patients were included and gave written, informed consent for participation in the study. Out of the initial study population, 29 patients and 10 patients respectively were available to provide follow-up swabs 2 and 4 weeks after HD initiation. Baseline characteristics of the study population are given in Table 1. The majority (69.7%) were male, and 32.5% were diabetic. Only a small proportion had history of broad-spectrum antibiotic use (11.6%) or hospitalization (16.2%) in the past 3 to 6 months, respectively.
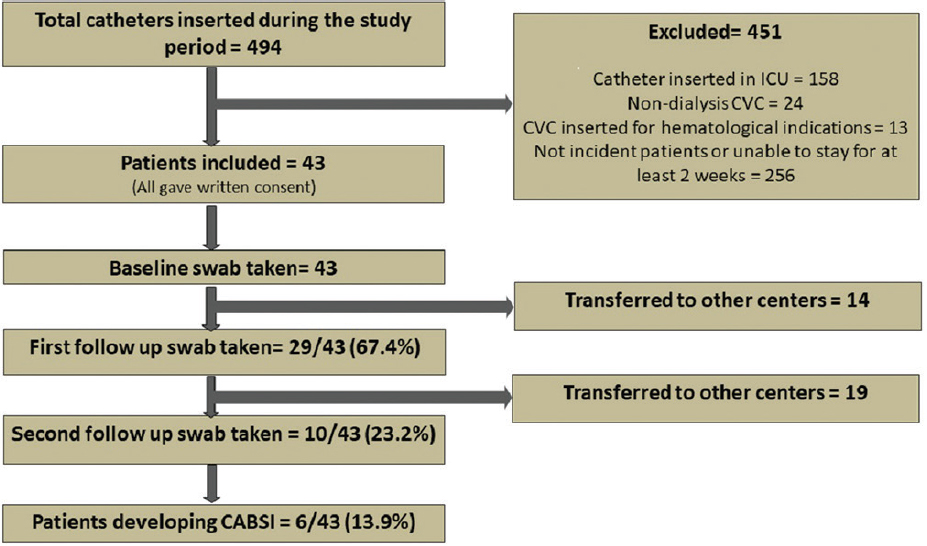
- STROBE diagram for the study
| Variables | Mean±SD or % (n=43) |
|---|---|
| Age (years) | 45±14 |
| Male (%) | 69.7 |
| BMI (kg/m2) | 23.8±5.9 |
| Mean Charlson’s Comorbidity Index | 3.3±1.6 |
| Diabetes (%) | 32.5 |
| Median interval between CKD diagnosis and HD initiation (months) | 12 (IQR 1, 41) |
| CKD EPI eGFR at HD start (ml/min/1.73 m2) | 5±2 |
| Broad spectrum antibiotic use in the last 3 months (%) | 11.6 |
| Hospitalization in the last 6 months (%) | 16.2 |
| Cause of ESRD (%) | Diabetic nephropathy-20.9 |
| Chronic glomerulonephritis-9.3 | |
| Tubulointerstitial disease-4.7 | |
| Obstructive uropathy-2.3 | |
| Unknown-62.8 | |
| Emergency HD initiation (%) | 51.1 |
| Functional AVF at dialysis initiation (%) | 0 |
| HD frequency-thrice Weekly (%) | 100 |
| Site of access-IJV (%) | 95.3 |
| Type of access-Temporary non-tunneled (%) | 67.4 |
| Position of access-Right (%) | 97.6 |
| Median duration of follow-up | 29 Days (IQR 12, 60) |
BMI=Body Mass Index; CKD=Chronic kidney disease; HD=Haemodialysis; CKD EPI eGFR=estimated glomerular filtration rate calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation; ESRD=End stage renal disease; AVF=arteriovenous fistula; IJV=internal jugular vein
Proportion of patients colonized with MDR bacteria prior to HD initiation
At least one organism was cultured from 96.5% of the swabs taken at baseline, and 47.4% swabs grew MDR organisms. The majority of colonizing organisms were gram-positive (91.2%), with methicillin-resistant S. aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) being the most common (28.3% and 26.7% of all organisms cultured, respectively). MDR bacterial colonization, MRSA colonization, and MDR gram-negative colonization at one or more sites on the baseline set of swabs was seen in 76.7%, 69.7%, and 9.3% patients respectively [Table 2]. In 30 patients with MRSA colonization, MRSA was isolated from both nasal and axillary swabs in 13 (43.3%), axillary alone in 12 (40%), nasal alone in 4 (13.3%), and nasal and proposed exit site in 1 patient (3.3%). MDR gram-negative colonization was seen only in the axilla.
| Organism | Nasal colonization, % (n=54 from 43 swabs) | Axillary colonization, % (n=132 from 86 swabs) | Exit Site colonization, % (n=54 from 43 swabs) | Overall colonization, % (n=240 from 172 swabs) | Patients colonized, % (n=43) |
|---|---|---|---|---|---|
| MSCoNS | 18.5 | 17.4 | 22.2 | 18.7 | 55.8 |
| MRCoNS | 18.5 | 12.8 | 20.4 | 15.8 | 55.8 |
| MSSA | 25.9 | 24.2 | 33.3 | 26.7 | 69.7 |
| MRSA | 33.3 | 28.0 | 24.1 | 28.3 | 69.7 |
| GNB | 0 | 9.8 | 0 | 5.4 | 13.9 |
| MDRGNB | 0 | 6.1 | 0 | 3.3 | 9.3 |
n in each column refers to the total number of organisms cultured from that site, while in the last column it refers to the total number of patients. MSCoNS=Methicillin Sensitive Coagulase Negative Staphylococcus; MRCoNS=Methicillin Resistant Coagulase negative Staphylococcus; MSSA=Methicillin Sensitive S. aureus; MRSA=Methicillin Resistant S. aureus; GNB=Gram negative bacilli; MDRGNB=Multidrug Resistant Gram Negative Bacilli
Factors associated with colonization by MDR bacteria prior to HD initiation
Females, diabetics, those with history of broad-spectrum antibiotic use in the last 3 months and those with higher comorbidity index showed a trend towards colonization by MDR organisms prior to HD initiation, but these factors did not reach statistical significance [Table 3].
| Variables | No colonization by drug resistant organisms (n=9) Mean +SD or Median (IQR) or % | Colonization by drug resistant organisms (n=34) Mean +SD or Median (IQR) or % | P |
|---|---|---|---|
| Age (years) | 40+9.7 | 47+14.6 | 0.91 |
| Male (%) | 88.9 | 64.7 | 0.16 |
| BMI (kg/m2) | 23.1+3.5 | 24.1+6.5 | 0.67 |
| Mean Charlsons Comorbidity Index | 2.67+1.1 | 3.56+1.7 | 0.14 |
| Diabetes (%) | 11.1 | 38.2 | 0.14 |
| Median interval between CKD diagnosis and initiation of HD (Months) | 18 (5,24) | 10 (1,41) | 0.58 |
| Antibiotic Use in the last 3 months (%) | 0 | 14.7 | 0.22 |
| Hospitalization in the last 6 months (%) | 11.1 | 17.6 | 0.64 |
| Median Serum Albumin | 3.8 (3.4,4.1) | 3.5 (2.9,3.9) | 0.26 |
| Median Serum Ferritin | 508.9 (261.1, 562.6) | 424 (260.4, 674.1) | 0.87 |
| CKD EPI eGFR at dialysis initiation (ml/min/1.73 m2) | 5+2.0 | 5+2.2 | 0.72 |
BMI=Body Mass Index; CKD=Chronic kidney disease; HD=Haemodialysis; CKD EPI eGFR=estimated glomerular filtration rate calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation
Change in colonization pattern over time
Supplementary Tables 1 and 2 show the colonization pattern observed on swabs obtained 14-21 days and 28-35 days after the baseline swab. The only significant change seen was a decrease in patients colonized by methicillin-sensitive coagulase-negative staphylococcus (MSCoNS) between baseline and 14-21 days [P = 0.03, Table 4]. In terms of organisms cultured on swabs, methicillin-resistant CoNS (MRCoNS) on swabs increased significantly from baseline to 14-21 days (P = 0.02) and baseline to 28-35 days (P = 0.001), with a consequent fall in colonization by MSCoNS [p < 0.001, Supplementary Table 3]. There was no significant change in patients colonized by, or swabs positive for, MRSA over time.
| Organism | Nasal, % (n=34 from 29 swabs) | Axillary, % (n=77 from 58 swabs) | Exit Site, % (n=11 from 29 swabs) | Overall, % (n=122 from 116 swabs) | Patients colonized (%) (n=29) |
|---|---|---|---|---|---|
| MSCoNS | 8.82 | 3.90 | 0 | 4.92 | 20.7 |
| MRCoNS | 35.29 | 23.38 | 18.18 | 26.23 | 65.5 |
| MSSA | 26.47 | 20.78 | 9.09 | 21.31 | 58.6 |
| MRSA | 26.47 | 40.26 | 45.45 | 36.89 | 82.7 |
| GNB | 0 | 5.19 | 27.27 | 5.74 | 24.1 |
| MDRGNB | 0 | 5.19 | 0 | 3.28 | 13.8 |
n in each column refers to the total number of organisms cultured from that site, while in the last column it refers to the total number of patients colonized. MSCoNS=Methicillin Sensitive Coagulase Negative Staphylococcus; MRCoNS=Methicillin Resistant Coagulase negative Staphylococcus; MSSA=Methicillin Sensitive S. aureus; MRSA=Methicillin Resistant S. aureus; GNB=Gram negative bacilli; MDRGNB=Multidrug Resistant Gram Negative Bacilli
| Organism | Nasal, % (n=8 from 10 swabs) | Axillary, % (n=28 from 20 swabs) | Exit Site, % (n=4 from 10 swabs) | Overall, % (n=40 from 40 swabs) | Patients colonized (%) n=10 |
|---|---|---|---|---|---|
| MSCoNS | 0 | 10.71 | 0 | 7.50 | 30 |
| MRCoNS | 62.5 | 32.14 | 25 | 37.50 | 80 |
| MSSA | 12.5 | 10.71 | 25 | 12.50 | 40 |
| MRSA | 25 | 39.29 | 50 | 37.50 | 60 |
| GNB | 0 | 7.14 | 0 | 5.00 | 10 |
| MDRGNB | 0 | 0 | 0 | 0 | 0 |
n in each column refers to the total number of organisms cultured from that site while in the last column it refers to the total number of patients colonized. MSCoNS=Methicillin Sensitive Coagulase Negative Staphylococcus; MRCoNS=Methicillin Resistant Coagulase negative Staphylococcus; MSSA=Methicillin Sensitive S. aureus; MRSA=Methicillin Resistant S. aureus; GNB=Gram negative bacilli; MDRGNB=Multidrug Resistant Gram Negative Bacilli
| Organism | Patients colonized at baseline, % (N1=43) | Patients colonized at 14-21 days, % (N2=29) | Patients colonized at 28-35 days, % (N3=10) |
|---|---|---|---|
| MSCoNS | 55.8 | 20.6* | 30 |
| MRCoNS | 55.8 | 65.5 | 80 |
| MSSA | 69.7 | 58.6 | 40 |
| MRSA | 69.7 | 82.7 | 60 |
| GNB | 13.9 | 24.1 | 10 |
| MDRGNB | 9.3 | 13.7 | 0 |
*P=0.03 between N1 and N2. MSCoNS=Methicillin Sensitive Coagulase Negative Staphylococcus; MRCoNS=Methicillin Resistant Coagulase Negative Staphylococcus; MSSA=Methicillin Sensitive S. aureus; MRSA=Methicillin Resistant S. aureus; GNB=Gram negative bacilli; MDRGNB=Multidrug Resistant Gram Negative Bacilli
| Organism | Bacteria cultured from baseline swabs, % (n=240) | Bacteria cultured from swabs at 14-21 days, % (n=122) | Bacteria cultured from swabs at 28-35 days, % (n=40) |
|---|---|---|---|
| MRSA | 28.3 | 36.9 | 37.5 |
| MSSA | 26.6 | 21.3 | 12.5 |
| MRCoNS | 15.8 | 26.2* | 37.5*** |
| MSCoNS | 18.7 | 4.9** | 7.5 |
| MDRGNB | 3.3 | 3.3 | 0 |
| GNB | 5.4 | 5.7 | 5.0 |
n in each column refers to the total number of organisms cultured from all sites at each time point. MRSA=Methicillin Resistant S. aureus; MSSA=Methicillin Sensitive S. aureus; MRCoNS=Methicillin Resistant Coagulase negative Staphylococcus; MSCoNS=Methicillin Sensitive Coagulase Negative Staphylococcus, MDRGNB=Multidrug Resistant Gram Negative Bacilli; GNB=Gram negative bacilli; *P=0.02 between baseline and 14-21 days, **P<0.001 between baseline and 14-21 days. ***P=0.001 between baseline and 28-35 days
Persistent vs. transient colonization by MDR bacteria
A total of 29 patients had at least two consecutive sets of swabs obtained. Of these, 23 patients (79.3%) were classified as persistent colonizers. Of the persistent colonizers, 19/23 (82.6%) were persistent intrinsic colonizers. The most common MDR organism associated with persistent colonization was MRSA. Of the 38 sites at which persistent colonization was documented in the 23 persistent colonizers, MRSA was cultured at 25/38 sites (65.7%), while MRCoNS was cultured in the rest. The most common site of persistent colonization was the axilla (n = 19, 50%). In persistent acquired colonizers (n = 4), who formed a subgroup of the persistent colonizers, the most common organism cultured was MRCoNS in 75%, and the most common site was the axilla (100%). There was no persistent colonization by MDR gram-negative bacteria. Four patients acquired MRSA at the exit site, and in all four cases the strain was the same as that which had colonized the axilla previously.
Correlation between colonizing bacteria and bacteria causing CABSI
Six patients developed a CABSI during the follow-up period, accounting for a CABSI incidence rate of 3.7 per 1000 patient days. Of these 6 patients, 3 were persistent colonizers (3/3 with MRSA, 2/3 with MRCoNS); however, neither of these organisms caused the CABSI in each case. Instead, 6 of CABSIs were caused by gram-negative organisms (Klebsiella pneumoniae– 2, Enterobacter – 2, Pseudomonas aeruginosa– 1, MRCoNS – 1). In only 1/6 cases (16.6%) was the strain of bacteria (Klebsiella pneumoniae) causing CABSI identical to that which had transiently colonized the skin on a previous swab. The median time between last swabs taken and diagnosis of CABSI was 13.5 days. Persistent colonization was not associated with CABSI (P = 0.24).
Discussion
A 2014 metanalysis put the pooled estimate of MRSA colonization in prevalent dialysis (HD and PD) patients at 6.2%. This estimate varied depending on geographical region (4% in Europe, 7.9% in USA and 10.3% in Asia), mode of dialysis (7.2% in HD vs. 1.3% in PD) and setting (14.2% for inpatients vs. 5.4% for outpatients). In the light of these estimates, the finding that incident outpatient Indian HD patients, presumably a low-risk population for MRSA colonization, had such a high prevalence of MRSA colonization prior to HD initiation, is disquieting. There is limited data on the prevalence of community-acquired MRSA (CA-MRSA) in India: it is higher in the urban population (3.9% in rural children vs. 5% in slum children) and increases with age (2.1% in children aged 5-9 years, 5.8% in children aged 10-15 years and 5.3% in healthy urban Indian adults).[1213] This is much higher than the pooled prevalence in the general population (1.3%) and in the general population without health care contacts (0.82%) in the USA.[14] We can therefore conclude that the high baseline colonization with MDR organisms is at least partly explained by high community colonization rates.
A recent metanalysis concluded that the prevalence of MRSA colonization did not vary between studies that swabbed only the nares and those that also swabbed extra-nasal sites;[15] however, our findings suggest that 40% with MRSA colonization may have been missed if a restricted swabbing policy had been followed. Although females, diabetics, those with history of broad-spectrum antibiotic use in the last 3 months and those with higher comorbidity index showed a propensity for higher colonization by MDR organisms at baseline, none of these factors reached statistical significance, probably due to the small sample size. However, all these are recognized risk factors for MRSA colonization in the community.[161718]
The high degree of persistent colonization by MRSA and MRCoNS is along expected lines, given the ability of these organisms to form biofilms that confer on them the added survival advantages of surface adherence and resistance to host immune surveillance mechanisms and antimicrobial agents.[19] Persistent colonization by MRSA has been linked to an 11.5 times higher risk of subsequent MRSA infections in prevalent HD patients.[15] It is not surprising, therefore, that an inordinate amount of time, money, and reams of journal pages have been devoted to the screening and treatment of MRSA carriage in dialysis patients. We would argue, based on our study findings, that, in the Indian subcontinent, this would amount to barking up the wrong tree.
Notwithstanding high baseline and persistent MRSA and MRCoNS colonization, the majority of CABSIs were gram-negative. This is in line with previously published data from other centres in India,[2021] including ours.[7] Our data suggests that baseline colonization with gram-negative bacteria is low, and gram-negative bacteria did not show persistent colonization. Only one of the six (1/6) patients who developed a CABSI had the culprit organism on a previous swab. Thus, routine screening and surveillance swabs may predict only 17% of subsequent CABSIs in India.
The remaining 6 of the 7 (83%) of CABSIs in our study were caused by organisms that had not been isolated on swabs obtained prior to the CABSI event, indicating that they were acquired. Since we do not reuse dialyzers and standards for water purity were strictly maintained throughout the study period, these sources of gram-negative bacteremia are unlikely. There are therefore two remaining routes of infection. One; endogenous infection via sites commonly associated with gram-negative colonization (eg. hands, groin, perineum) due to poor hand hygiene by patients, second; exogenous infection via fomite transmission from the dialysis facility or poor adherence to hand hygiene among dialysis center care providers. It is imperative that all dialysis units provide patients standardized education on hand hygiene, catheter care, risks related to catheter use, signs of catheter-related infection, and how to access care when away from the dialysis unit.[22] Given that intensive hand hygiene protocols restricted to dialysis patients alone have been associated with modest benefit in reducing CABSI incidence in India,[23] it is likely that hand hygiene adherence among dialysis staff may be a crucial determinant of CABSI risk, though the present study design does not provide direct evidence to support this conclusion. The Center for Disease Control has put forward a list of core interventions to reduce the incidence of dialysis-related bloodstream infections.[24] Apart from patient education, it recommends that all dialysis staff be trained in infection control, and vascular access care and competency evaluation be carried out upon hire and every 6-12 months thereafter. Monthly hand hygiene audits, and quarterly audits on vascular access care and catheter access technique (connecting, disconnecting, dressing) are also recommended. Results of all audits should be shared with the clinical staff.
It is also important to address barriers to the creation of a permanent vascular access that results in over 90% of Indian dialysis patients commencing HD with a temporary vascular access.[3] Delayed referral to a nephrologist is the first roadblock, however even timely referral to a nephrologist does not guarantee vascular access creation[25] unless nephrologists refer patients for vascular access in a timely fashion.[26] This is crucial because Indian CKD patients perceive nephrologists as key decision-makers with respect to when and how they should initiate renal replacement therapy[27] and are unlikely to opt for vascular access placement unless advised to do so. This lacuna can be filled if all nephrology services incorporate a nurse-practitioner-driven CKD education program[28] supervised by nephrologists, since patient dialysis knowledge is directly linked to construction of a permanent vascular access.[29]
Strengths and limitations
This study provides the first estimate of skin colonization by MDR organisms among incident HD patients in the subcontinent. We adopted rigorous inclusion criteria, our subjects hailed from all parts of India (85% hailed from other states), we obtained swabs from the nares and extra-nasal sites, followed patients prospectively, and adhered to established standards for swab collection, processing and culture. Our study results are therefore robust and broadly reflective of incident HD patients in the subcontinent.
This study has several limitations. The sample size is small because we chose to focus exclusively on incident HD patients who were likely to follow-up at our centre for at least 2 weeks in order to assess the effect of colonization on CABSI incidence. Since ours is a tertiary referral center with a majority hailing from other parts of the country, this resulted in a high attrition rate on follow-up. Although MRSA colonization rates do not differ between studies that performed one versus multiple screenings,[15] we cannot rule out that attrition may have affected the observed rate of colonization by gram-negative organisms. Given that the majority of CABSIs were gram negative, swabs from the hand, groin and/or perineum may have been useful to determine if gram-negative bacteremia resulted from endogenous transfer of gram-negative bacteria from these sites to the exit site due to poor patient hand hygiene practice. We did not obtain swabs from the dialysis environment or dialysis unit health care workers to determine if transfer from these reservoirs contributed to gram-negative CABSIs. Lastly, the results of our study may not be generalizable to other countries where the population microbial resistance dynamics, personal and/or dialysis unit hygiene practices may differ.
Conclusion
Approximately three-fourths of the incident HD population in India is colonized by MDR bacteria prior to dialysis initiation, with MRSA being the most common isolate. Persistent colonization by MRSA and/or MRCoNS is seen in the majority, yet CABSIs are predominantly gram negative. Approximately 16% of CABSIs are caused by skin colonizing organisms. Studies to explore the role of endogenous or exogenous luminal contamination as a cause for CABSIs in the Indian subcontinent are warranted.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was funded by an Internal Fluid Research Grant from Christian Medical College, Vellore. [IRB Minute number 10683 (OBSERVE) dated 1st June 2017, Account number 22Z232].
Conflicts of interest
There are no conflicts of interest.
References
- Practice pattern of hemodialysis among end-stage renal disease patients in Rural South India: A single-center experience. Saudi J Kidney Dis Transpl. 2017;28:1150-6.
- [Google Scholar]
- Hemodialysis outcomes and practice patterns in end-stage renal disease: Experience from a tertiary care hospital in Kerala. Indian J Nephrol. 2017;27:51-7.
- [Google Scholar]
- A cross-sectional study of dialysis practice-patterns in patients with chronic kidney disease on maintenance hemodialysis. Saudi J Kidney Dis Transpl. 2015;26:1050-6.
- [Google Scholar]
- Referral pattern of patients with end-stage renal disease at a public sector hospital and its impact on outcome. Natl Med J India. 2011;24:208-13.
- [Google Scholar]
- Associations between hemodialysis access type and clinical outcomes: A systematic review. J Am Soc Nephrol. 2013;24:465-473.
- [Google Scholar]
- Fast and furious: A retrospective study of catheter-associated bloodstream infections with internal jugular nontunneled hemodialysis catheters at a tropical center. Clin Kidney J. 2019;12:737-44.
- [Google Scholar]
- Nasal screening for staphylococcus aureus – Daily routine with improvement potentials. PLoS One. 2014;9:e89667.
- [Google Scholar]
- Koneman's Color Atlas and Textbook of Diagnostic Microbiology. (6th ed). Lippincott Williams & Wilkins; 2006.
- [Google Scholar]
- Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-81.
- [Google Scholar]
- Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central Line Associated Bloodstream Infection) Published online January 2018 Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf
- [Google Scholar]
- A community-based study on nasal carriage of Staphylococcus aureus. Indian J Med Res. 2009;130:742-8.
- [Google Scholar]
- Methicillin-resistant staphylococcus aureus prevalence in community in the east Delhi area. Jpn J Infect Dis. 2003;56:54-6.
- [Google Scholar]
- Community-acquired methicillin-resistant Staphylococcus aureus: A meta-analysis of prevalence and risk factors. Clin Infect Dis Off Publ Infect Dis Soc Am. 2003;36:131-9.
- [Google Scholar]
- Meta-analysis of methicillin-resistant staphylococcus aureus colonization and risk of infection in dialysis patients. J Am Soc Nephrol. 2014;25:2131-41.
- [Google Scholar]
- Prevalence of and risk factors for colonization by methicillin-resistant staphylococcus aureus among adults in community settings in Taiwan. J Clin Microbiol. 2009;47:2957-63.
- [Google Scholar]
- Risk factors for colonization with methicillin-resistant staphylococcus aureus (MRSA) in patients admitted to an urban hospital: Emergence of community-associated MRSA nasal carriage. Clin Infect Dis. 2005;41:159-66.
- [Google Scholar]
- Predictors of methicillin-resistant staphylococcus aureus colonization at hospital admission. Am J Infect Control. 2013;41:1043-7.
- [Google Scholar]
- Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318-22.
- [Google Scholar]
- Microbiology of non-tunnelled catheter-related infections. J Clin Diagn Res. 2016;10:DC24-8.
- [Google Scholar]
- Time to revisit the use of nontunneled dialysis vascular catheters even in cost-limited setting. Indian J Nephrol. 2018;28:406-7.
- [Google Scholar]
- CDC. Guidelines for prevention of intravascular Catheter-related infections (2011) Published November 21, 2017 Available from: https://www.cdc.gov/infectioncontrol/guidelines/bsi/index.html
- [Google Scholar]
- The effectiveness of personal hygiene practices on non-cuffed central vein catheter-related infection in patients undergoing hemodialysis: A randomized controlled trial. Indian J Nephrol. 2019;29:267-71.
- [Google Scholar]
- Core Interventions. Published April 25, 2019 Available from: https://www.cdc.gov/dialysis/prevention-tools/core-interventions.html
- [Google Scholar]
- Why do patients known to renal services still undergo urgent dialysis initiation. A cross-sectional survey? Nephrol Dial Transplant. 2007;22:3240-5.
- [Google Scholar]
- Initiation of maintenance hemodialysis through central venous catheters: Study of patients’ perceptions based on a structured questionnaire. BMC Nephrol. 2019;20:270.
- [Google Scholar]
- “Why I Chose hemodialysis over peritoneal dialysis”: An opinion survey among in-center hemodialysis patients. Perit Dial Int. 2018;38:305-8.
- [Google Scholar]
- Implementing patient education in the CKD clinic. Adv Chronic Kidney Dis. 2013;20:320-5.
- [Google Scholar]
- Patient dialysis knowledge is associated with permanent arteriovenous access use in chronic hemodialysis. Clin J Am Soc Nephrol. 2009;4:950-6.
- [Google Scholar]







