Translate this page into:
Strategies to Circumvent Discrepancies in Pre-Transplant Donor Specific Antibodies Workup
Corresponding author: Ranjana W. Minz, Department of Immunopathology, Post Graduate Institute of Medical Education and Research (PGIMER), India. E-mail: rwminz.minz88@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rani L, Aggarwal R, Kumar M, Ramachandran R, Sharma A, Minz RW. Strategies to Circumvent Discrepancies in Pre-Transplant Donor Specific Antibodies Workup. Indian J Nephrol. 2025;35:309-10. doi: 10.25259/IJN_60_2024
Dear Editor,
Accurate assessment of donor-specific antibodies (DSAs) plays a pivotal role in pre-transplant evaluation to mitigate the risk of graft rejection.1 However, a challenging dilemma arises when DSA-SAB results show positive results, but other assays, such as complement-dependent cytotoxic crossmatch (CDC-XM) and flow crossmatch (FC-XM) provide negative results.
We present two cases to illustrate the diagnostic challenges encountered during the pre-transplant workup:
Case 1: A 31-year-old man underwent a pre-transplant evaluation including CDC-XM, FC-XM, panel reactive antibody (PRA) testing, and DSA-SAB assay with his father as a donor. Despite negative results in CDC-XM and FC-XM, the DSA-SAB assay revealed unexpected weak-to-moderate positivity (MFI range = 1000-5000) against a wide range of HLA class I and class II antigens [Figure 1a and 1b]. PRA testing showed no-HLA sensitization [Figure 1c and 1d], but the test negative control (CON) values were high, indicating the nonspecific binding, which leads to false-positive tests. In such a scenario, PRA testing alongside DSA-SAB helps.2
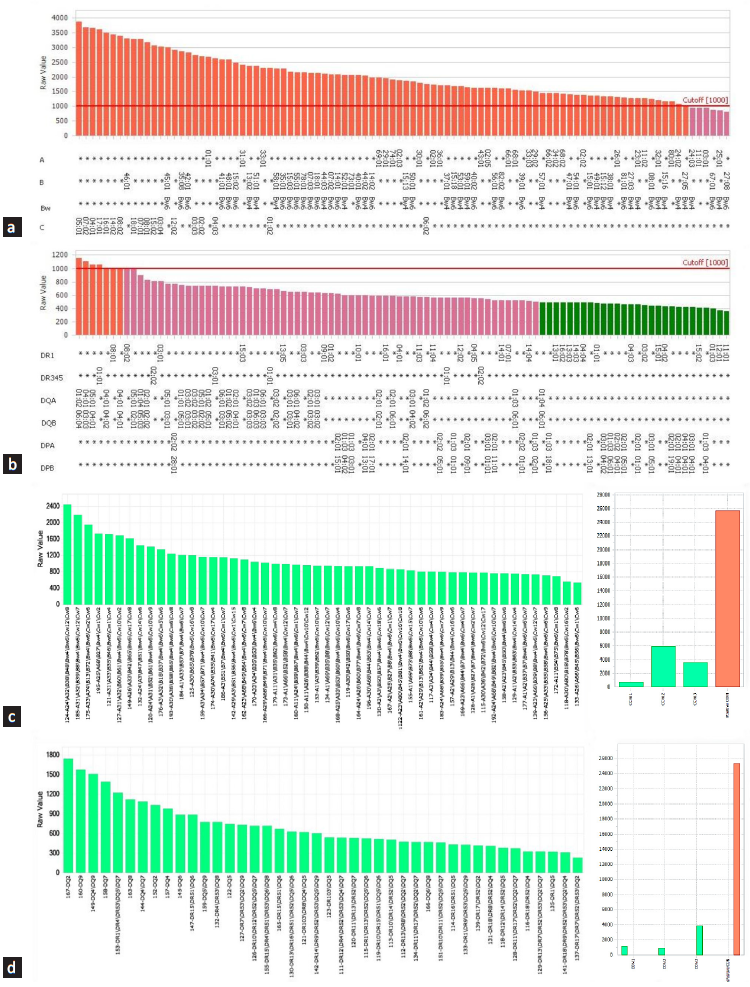
- Assessment of true or false anti-HLA antibodies: a case. Figure showing broad reactivity to (a) HLA-class I single antigen beads profile; (b) HLA-class II single antigen beads profile; (c) HLA-class I PRA beads profile; (d) HLA-class II PRA beads profile. Colored rectangles indicate the MFI of antibodies for corresponding HLA antigens. (a and b): Red color indicates MFI ≥ 1000, purple indicates MFI ≤ 1000 and dark green indicates MFI ≤ 500. (c and d): Green color indicates negative bead reactions for a particular HLA antigen, and orange indicates positive bead reactions. (Y-axis: MFI values, X-axis: SAB HLA specificity) HLA: Human leukocyte antigen, PRA: Panel reactive antibody.
Case 2: A 33-year-old man was planned for transplant with his sister as a donor. Patient exhibited HLA class II positivity for self-antigen DRB1*13:01, in DSA-SAB testing (Immucor). However, CDC-XM and FC-XM were negative. Repeat testing with another kit from a different vendor (One Lambda, Inc.) showed the absence of antibodies for self-antigen DRB1*13:01. Reported false positivity may occur due to the presence of antibodies to denatured antigens.2,3
We propose stepwise strategies to address these diagnostic challenges:
Patient history should be assessed thoroughly for sensitization events.
Conduct high-resolution typing and utilize multiple assays to determine true antibodies, including different platforms, solid-phase assays and kits from other vendors.
Perform epitope analysis to decipher antibody specificities.4
This letter emphasizes the importance of quality control, technical validation, and personalized patient-focused assessments in pre-kidney transplant evaluations.
Acknowledgment
We acknowledge Mr. Manoj Kumar and Mr. Heera Singh for technical assistance.
Conflicts of interest
There are no conflicts of interest.
References
- Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662-5.
- [CrossRef] [PubMed] [Google Scholar]
- Detecting Donor-Specific Antibodies: the Importance of sorting the wheat from the chaff. Hepatobiliary Surg Nutr. 2019;8:37.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Positive single-antigen bead assay with negative flow crossmatch in a renal transplant-A case report. Indian J Transplant. 2022;16:425-7.
- [CrossRef] [Google Scholar]
- Epitope analysis aids in transplant decision making by determining the clinical relevance of apparent pre-transplant donor specific antibodies (DSA) Ann Clin Lab Sci. 2019;49:50-6.
- [PubMed] [Google Scholar]






