Translate this page into:
Successful Reversal of Refractory Posttransplant Thrombotic Microangiopathy with Eculizumab
Corresponding author: Dr. Reetesh Sharma, Director and Head, Nephrology and Kidney Transplant Medicine, Asian Institute of Medical Sciences, Faridabad, India. E-mail: reeteshis@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Chaudhary S, Sharma R, Gupta S, Paikra S, Gupta M, Upadhyay BK, et al. Successful Reversal of Refractory Posttransplant Thrombotic Microangiopathy with Eculizumab. Indian J Nephrol. 2024;34:191–4. doi: 10.4103/ijn.ijn_345_22
Abstract
Posttransplant thrombotic microangiopathy (PT-TMA) can be caused by calcineurin inhibitors (CNIs), ischemic injury, infections, or antibody-mediated rejection (ABMR). Delayed recognition can result in allograft loss. We describe the first reported case of successful reversal of refractory PT-TMA with eculizumab in India. It highlights the importance of prompt diagnosis and benefit from an early initiation of eculizumab therapy in refractory cases.
Keywords
Eculizumab
refractory posttransplant TMA
thrombotic microangiopathy
Introduction
Posttransplant thrombotic microangiopathy (PT-TMA) is a rare devastating condition that can lead to poor patient and graft outcomes. Its incidence varies from 0.8% to 15%, with allograft loss in up to 33%–50%.1,2
The treatment requires correcting the cause. Withdrawal of calcineurin inhibitor (CNI) treatment and plasma exchange may not be sufficient to fully reverse the adverse effects of the alternative pathway of complement activation.2,3 Eculizumab has beenused to treat resistant de novo PT-TMA.4-6 We describe the successful use of eculizumab in a transplant recipient with native IgA nephropathy who developed immediate PT-TMA.
Case Report
A 16-year-old boy presented with nephritic nephrotic syndrome. His kidney biopsy revealed mesangial and focal endocapillary proliferative crescentic IgA nephropathy (M1E1S1T1C2). He was treated with steroids and pulse cyclophosphamide followed by mycophenolate sodium (MMF) and optimal renin angiotensin aldosterone system blockade. He progressed to end-stage kidney disease over 2 years and was initiated on maintenance hemodialysis. He underwent kidney transplantation with his father as the donor, and received thymoglobulin induction and tacrolimus, MMF, and steroids as maintenance immunosuppression.
Immediate posttransplant, the patient had good graft function but developed fever on postoperative day (POD) 1, and urine culture sent revealed growth of Klebsiella pneumoniae. His antibiotics were upgraded to ceftriaxone disodium edetate + sulbactam as per sensitivity and MMF was put on hold.
In view of persistent fever and decline in blood pressure, suspecting worsening sepsis, a second antibiotic (intravenous [IV] fosfomycin) was added on POD 4. and DJ stent was removed on POD 7.
Since the fever persisted, computed tomography chest and abdomen were done, but were unremarkable. Polymixin B was added at this time.
Finally, fever settled on POD 11, but he developed graft dysfunction after POD 12 (S. creatinine: 1.0 → 1.4 mg/dl). This was attributed to volume depletion and drug toxicity (fosfomycin + polymyxin B), hence hydration and antibiotic doses were optimized. He developed accelerated hypertension on POD 13, and was restarted on antihypertensives.
Patient experienced further worsening of graft function along with anemia and thrombocytopenia [Table 1]. On POD 17, there was elevated lactate dehydrogenase (LDH) level and the presence of schistocytes on peripheral smear, confirming microangiopathic hemolytic anemia. Hence a diagnosis of PT-TMA was made and tacrolimus was discontinued. He was pulsed with IV methylprednisolone (250 mg) for 3 days (patient was off MMF and CNIs).
| Presurgery | POD 0 | POD 3 | POD 10 | POD 12 | POD 17 | POD 19 | POD 25 | POD 28 | POD 35 | POD 42 | POD 49 | POD 60 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin, g/dl | 11.2 | 12.3 | 11.7 | 11.6 | 9.5 | 10.7 | 9.2 | 7.8 | 5.3 | 8.7 | 8 | 8.3 | 9.2 |
| Platelets, k/µl | 162 | 154 | 150 | 178 | 229 | 60 | 40 | 40 | 40 | 35 | 105 | 171 | 280 |
| Creatinine, mg/dl | 9.3 | 4.1 | 1 | 0.8 | 1.4 | 2.3 | 2.8 | 4.8 | 6 | 6 | 4.2 | 2.5 | 1.7 |
| LDH, IU/l | 232 | 518 | 539 | 2184 | 4140 | 1613 | 1420 | 516 | 472 | 393 | |||
| Tacrolimus, ng/ml | 8.7 | 10.5 | 8.6 | ||||||||||
| Infectious workup | EBV IgG: positive CMV IgG: positive HIV: negative |
CMV DNA PCR: negative, BK virus DNA PCR: negative | |||||||||||
| Autoimmune workup | Coombs: negative ANA: negative Anti-CFH and CFI antibody: negative |
||||||||||||
| Clinical exome study Gene of coding region covered (%) | ADMTS13 (100%)/DGKE (100%)/THBD (100%)/C3 (100%)/CFB (100%)/CFH (100%)/CFHR1 (93.81%)/CFHR2 (70.64%)/CFHR3 (97.37%)/CFHR4 (100%)/CFHR5 (100%)/CFI (100%) | ||||||||||||
| Start of treatment | PLEX+IV IG first session | PLEX+IV IG fifth session | Eculizumab | Eculizumab | Eculizumab | Eculizumab | |||||||
CMV=cytomegalovirus, EBV=Epstein–Barr virus, HIV=human immunodeficiency virus, IG=immunoglobulin, IV=intravenous, LDH=lactate dehydrogenase, PCR=polymerase chain reaction, POD=postoperative day
Since allograft biopsy could not be performed due to thrombocytopenia, LUMINEX SAB-DSA class I and II was sent (to rule out antibody-mediated rejection [ABMR]-induced TMA) which was negative. The PLASMIC (platelet count, combined hemolysis variable, absence of active cancer, absence of stem cell transplant, mean corpuscular volume, international normalized ratio, creatinine) score was 4, consistent with a low likelihood of thrombotic thrombocytopenic purpura, which was later confirmed by ADAMTS13 activity of 31.3%. Coombs test and anti nuclear antibody tests were also negative. Workup for bacterial infections (blood and urine culture), cytomegalovirus polymerase chain reaction (PCR), BK virus PCR, and parvovirus IgM were negative. Complement factor assay and gene sequencing were sent to rule outatypical hemolytic syndrome (aHUS).
Due to persistent TMA, despite stopping tacrolimus and pulse steroids, plasma exchange was initiated on POD 19. Five sessions of plasma exchange were done over the next week along with transfusion three units of PRBCs because of worsening hemolysis.
Despite these interventions there was no improvement in hematological or renal parameters. He was also dialysed once because of volume overload.
After discussion with the family, eculizumab treatment [Figure 1]. was given at a dose of 900 mg weekly for a total off our doses along with cephalosporin prophylaxis. After the second dose, hemoglobin and platelet count improved, and S. creatinine level decreased.
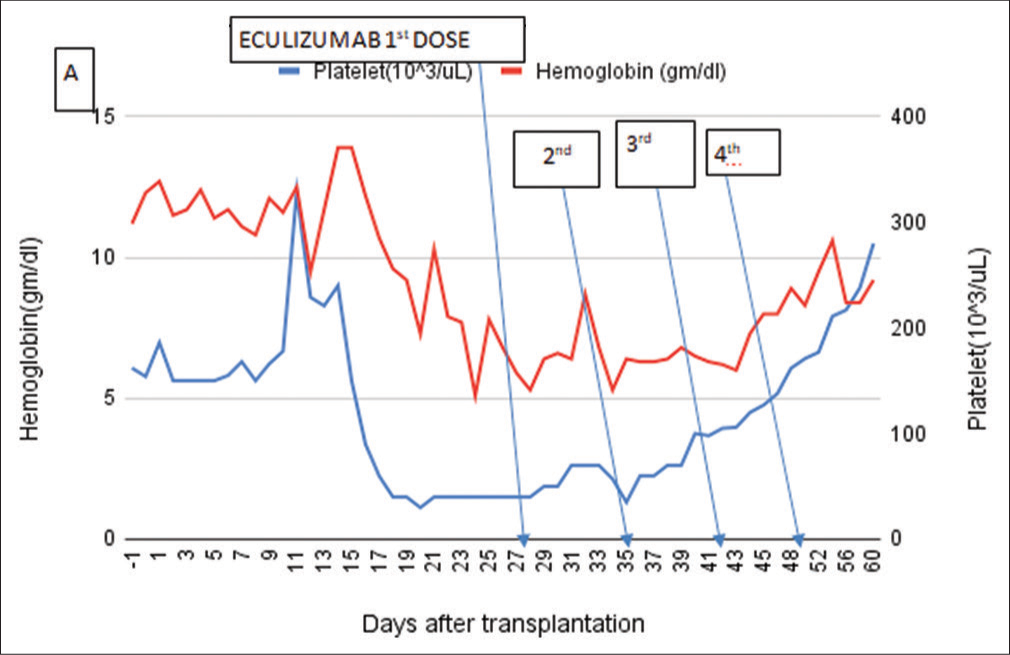
- Laboratory parameters show improvement after complement inhibition with eculizumab on postoperative day 28, with hemoglobin and platelet, returning to normal range. Arrow marks eculizumab therapy.
Once thrombocytopenia improved, the patient underwent allograft biopsy on POD 45, which confirmed the diagnosis of TMA with acute tubular necrosis.
Immunosuppression was maintained with steroids and MMF and cyclosporin was added later.
The gene sequencing results, available later were normal [Table 1]. Anti-Complement Factor H and Anti Complement Factor I CFH and CFI autoantibodies (by enzyme-linked immunosorbent assay [ELISA] method) were negative. Hence, eculizumab was stopped after four doses.7 One year after kidney transplantation, our patient has stable graft function with no relapse of TMA.
Discussion
The development of hemolytic anemia and thrombocytopenia post–kidney transplantation should raise concern for a microangiopathic process. The diagnosis of PT-TMA poses a diagnostic dilemma since a multitude of reasons, such as ischemia reperfusion injury, use of CNIs, or ABMR, alone or together can cause delayed graft function.8,9
PT-TMA results from endothelial damage due to unregulated complement activation and requires clinicopathologic correlation.10 Allograft biopsy can be confirmatory but carries a high risk of bleeding.
In our patient, TMA occurred in the second week posttransplantation [Figures 2 and 3] and failed to respond to discontinuation of tacrolimus and plasmapheresis therapy, raising the possibility of aHUS. However, his native disease being biopsy-proven IgA nephropathy reduced the likelihood of recurrence of aHUS. In addition, we performed complement studies and gene sequencing to rule out this possibility.
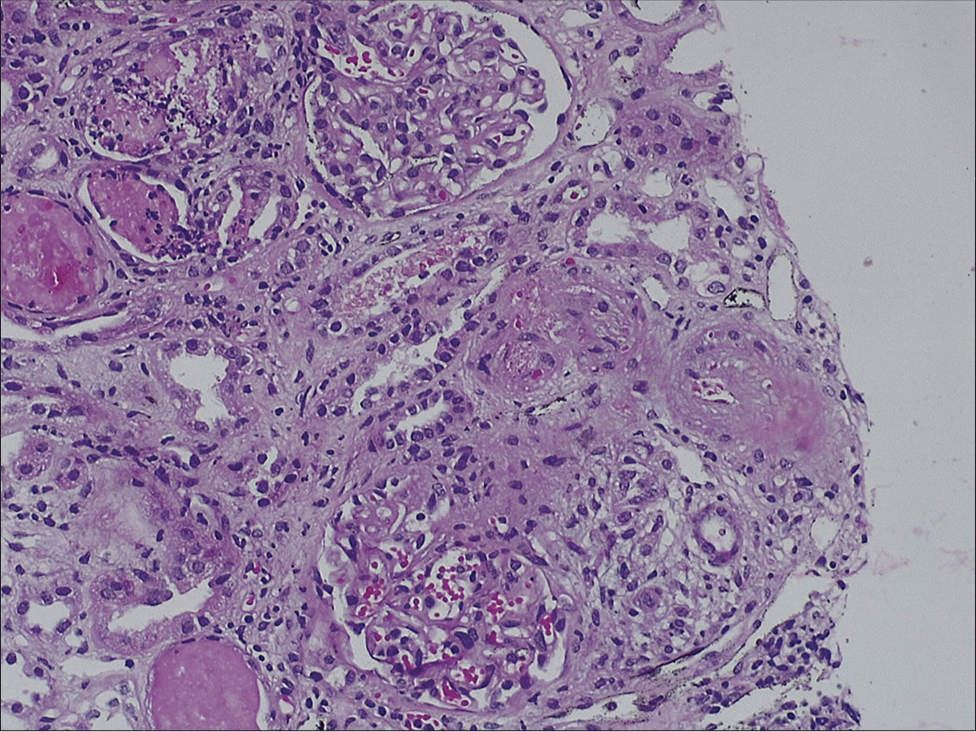
- Light microscopy of the kidney biopsy specimen (hematoxylin and eosin stain) showing necrosis of arterial and arteriolar walls with RBC fragmentation and luminal thrombotic occlusion (TMA) with ischemic mesangiolysis of glomerular tufts. Stain for C4d was negative along peritubular capillaries (4.69 × 6.76 cm) RBC = red blood cell, TMA = thrombotic microangiopathy.
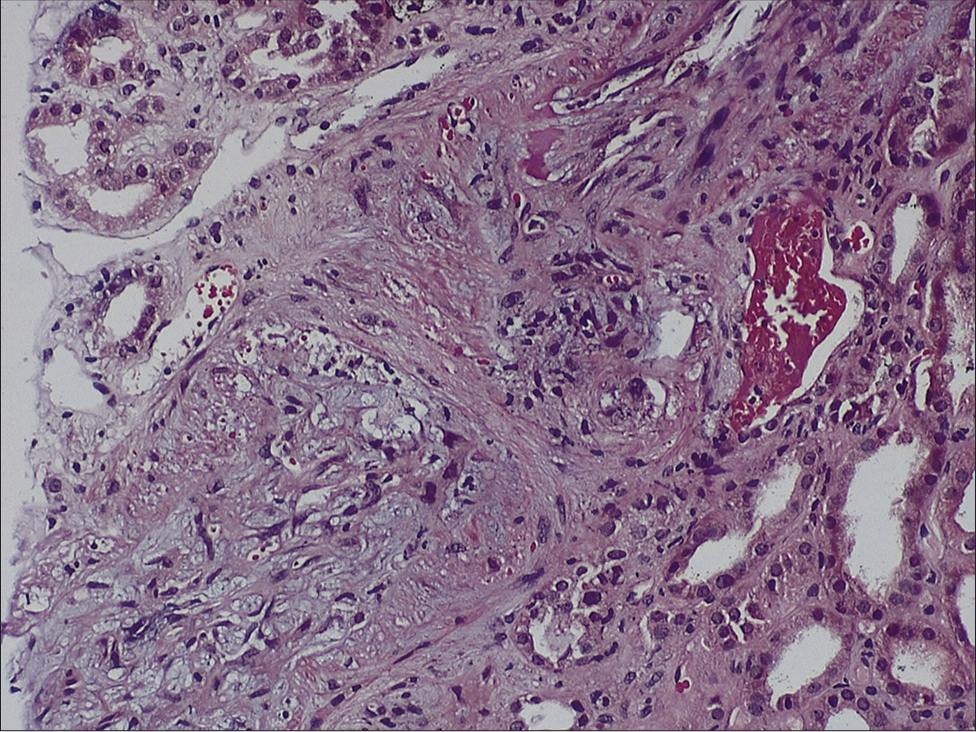
- Light microscopy of the kidney biopsy specimen (hematoxylin and eosin stain) showing necrosis of arterial and arteriolar walls with RBC fragmentation and luminal thrombotic occlusion (TMA) with ischemic mesangiolysis of glomerular tufts. Stain for C4d was negative along peritubular capillaries (4.55 × 7.49 cm) RBC = red blood cell, TMA = thrombotic microangiopathy.
An underlying predisposing genetic mutation can be identified in ~30% of patients with PT-TMA, and use of conventional therapies such as plasmapheresis can be ineffective in the absence of any quantitative defects in ADAMTS13 or inhibitors of complement pathway or ABMR.11,12
In such cases, early use of eculizumab can reverse the complement activation and prevent further organ damage.13
In our patient, urinary tract infection possibly triggered the complement cascade on a background of tacrolimus therapy, leading to the development of TMA.
Eculizumab, available through a restricted access program, is a recombinant fully humanized monoclonal antibody targeted against human complement protein C5 and blocks generation of lytic C5b-9 membrane attack complex. In PT-TMA, a complement overactivation has been demonstrated even in patients without pathogenic complement protein variants.14
Cavero et al.15 reported 15 kidney transplant patients with PT-TMA. All but one withdrew the offending drug. Twelve patients also received plasma exchange PE (a mean of 5.46 sessions per patient, range 2–12) without improvements in S. creatinine (mean 4 mg/dl, 3.4–5.6). Eculizumab was started from 4 to 53 days after TMA detection, and a mean of 6.4 doses from two to 17 per patient were used. At the end of follow-up, mean S. creatinine was 1.7 mg/dl, with no graft losses or recurrences of TMA after eculizumab discontinuation.
It has been proposed that patients with pathogenic complement regulatory mutations should continue eculizumab indefinitely to prevent TMA relapse, although it might be safe to discontinue it after 6–12 months of treatment in patients with no genetic predisposition.15
The current data are not strong enough to expand the recommendation for the use of eculizumab in all patients with PT-TMA, but in resistant cases eculizumab is a good therapeutic option.16
Our case highlights the importance of prompt diagnosis of PT-TMA and that early initiation of eculizumab in refractory cases allows recovery of renal graft and hematologic parameters. Genetic testing should be incorporated in the workup of TMA to identify any predisposition and hence guide the duration of therapy.
To the best of our knowledge, this is the first reported case of successful reversal of refractory PT-TMA with eculizumab in India.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- De novo thrombotic microangiopathy in renal transplant recipients: A comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis. 2003;41:471-9.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis. 2003;42:1058-68.
- [CrossRef] [PubMed] [Google Scholar]
- Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481-508.
- [CrossRef] [PubMed] [Google Scholar]
- Eculizumab and belatacept for de novo atypical hemolytic uremic syndrome associated with CFHR3-CFHR1 deletion in a kidney transplant recipient: A case report. Transplant Proc. 2017;49:188-92.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of eculizumab therapy for atypical hemolytic uremic syndrome recurrence and antibody-mediated rejection progress after renal transplantation with preformed donor-specific antibodies: Case report. Transplant Proc. 2017;49:159-62.
- [CrossRef] [PubMed] [Google Scholar]
- Two cases of kidney transplantation-associated thrombotic microangiopathy successfully treated with eculizumab. Nephrology (Carlton). 2016;21(Suppl 1):35-40.
- [CrossRef] [PubMed] [Google Scholar]
- Eculizumab discontinuation in children and adults with atypical hemolytic-uremic syndrome: A prospective multicenter study. Blood. 2021;137:2438-49.
- [CrossRef] [PubMed] [Google Scholar]
- De novo thrombotic microangiopathy in renal allograft biopsies-role of antibodymediated rejection. Am J Transplant. 2010;10:1804-11.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed graft function in the kidney transplant. Am J Transplant. 2011;11:2279-96.
- [CrossRef] [PubMed] [Google Scholar]
- Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant Rev (Orlando). 2013;27:117-25.
- [CrossRef] [PubMed] [Google Scholar]
- Case series: Hemolytic uremic syndrome-another cause of transplant dysfunction. Transplant Proc. 2013;45:3284-8.
- [CrossRef] [PubMed] [Google Scholar]
- Complement mutation associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant. 2008;8:1694-701.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of patients with atypical haemolyticuraemic syndrome with native and transplanted kidneys treated with eculizumab: a pooled post hoc analysis. Transpl Int. 2017;30:1275-83.
- [CrossRef] [PubMed] [Google Scholar]
- Use of highly individualized complement blockade has revolutionized clinical outcomes after kidney transplantation and renal epidemiology of atypical hemolytic uremic syndrome. J Am SocNephrol. 2019;30:2449-63.
- [Google Scholar]
- Eculizumab in secondary atypical haemolyticuraemicsyndrome. Nephrol Dial Transplant. 2017;32:466-74.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of kidney transplant patients with atypical hemolytic uremic syndrome treated with eculizumab: A systematic review and meta-analysis. J Clin Med. 2019;8:919. doi: 10.3390/jcm8070919
- [CrossRef] [PubMed] [Google Scholar]







