Translate this page into:
Thrombotic Microangiopathy Secondary to Pancreatitis: A Diagnostic Enigma
Address for correspondence: Dr. Sahil Bagai, M.D, D. M (Nephrology), Consultant Nephrology, Max Superspeciality Hospital, Saket, Delhi - 110 017, India. E-mail: sahil.bagai@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The association between thrombotic microangiopathy (TMA) and pancreatitis is well known. However, TMA leading to pancreatitis is more common than the latter. TMA and renal failure are both poor prognostic markers in acute pancreatitis. TMA, if not managed timely, can lead to severe morbidity and mortality. We report a case of a young boy in whom decisive and timely diagnosis and management of TMA post pancreatitis helped in complete patient and renal recovery.
Keywords
Atypical HUS
plasmapheresis
renal failure
thrombotic
Introduction
Thrombotic microangiopathy (TMA) refers to a group of diseases that share the same clinical features and histology. They all encompass microangiopathic hemolytic anemia (MAHA) defined as hemoglobin (Hb) <10 g/dL with evidence of hemolytic anemia; thrombocytopenia (platelet count <150 × 109/L), and end-organ dysfunction that in majority cases presents as renal involvement.[12] Disseminated intravascular coagulation and malignant hypertension act as common masqueraders. TMA is classified into “primary” syndromes, which include thrombotic thrombocytopenic purpura (TTP), Shiga toxin-mediated TMA (ST-TMA), and TMA associated with complement dysregulation (atypical hemolytic uremic syndrome, (aHUS), and “secondary” syndromes that are associated with known precipitating factors.[3] TMA can precipitate acute pancreatitis in 2% cases, but acute pancreatitis preceding TMA is far less common.[4] The short interval described between the diagnosis of pancreatitis and the diagnosis of TMA, a median of 3 days, raises the hypothesis that the inflammatory consequence of pancreatitis has a direct impact on the pathogenesis of TMA. Our patient developed TMA on the third day of acute pancreatitis and recovered completely following plasma exchange.
Case
A 15-year-old boy presented to the emergency room (ER) with multiple episodes of vomiting, loose stools, and upper abdominal pain with radiation to the back. Next day, he developed respiratory distress and had a decrease in urine output for which he was dialyzed and referred to our center. He had serum creatinine of 0.9 mg/dL on the day of admission which increased to 4.2 mg/dL at the time of referral. On presentation, he was febrile with a pulse rate of 112/min and blood pressure of 110/60 mm Hg. Systemic examination was unremarkable except for severe guarding and rigidity of the abdomen. Blood investigation results were as follows: hemoglobin 8.0 g/dL, total leukocyte count (TLC) 11,600/cu mm, platelet count 40,000/cu mm, blood urea nitrogen 81 mg/dL, creatinine 8.3 mg/dL, amylase 737 U/L, lipase 464 U/L, total bilirubin 2.7 mg/dL, direct bilirubin 0.63 mg/dL, aspartate transferase (AST) 253 IU/L, alanine transferase (ALT) 116 IU/L, alkaline phosphatase (ALP) 228 IU/L, albumin 3.1 g/dL, and calcium 8.1 mg/d. Urine microscopy revealed protein 1+, Pus cells 10–12/hpf, and RBC 5–10/hpf L. His lipid profile was normal; dengue, malaria, and leptospira serology were negative. Antinuclear antibody and stool culture were negative, and complements (C3/C4) were normal. Ultrasound abdomen revealed bulky pancreas with normal size and echogenic bilateral kidneys. MRI abdomen done was suggestive of bulky, edematous pancreas consistent with acute interstitial pancreatitis with mild fluid collection and peripancreatic stranding [Figure 1]. He was managed conservatively for acute interstitial pancreatitis but had to be dialyzed in view of advanced azotemia and persistent anuria. He developed melena and his hemoglobin dipped to 4.7 g/dl. Upper gastrointestinal endoscopy done revealed diffuse gastritis and was managed with proton pump inhibitors. Hemolytic work up was sent in view of persistent fall in hemoglobin and platelet counts and reports were as follows: reticulocyte count 2.9%, lactate dehydrogenase (LDH) 4052 U/L, glucose 6 phosphate dehydrogenase 26.4, serum haptoglobin <30 mg/dl, and negative direct Coomb's test; peripheral smear showed multiple fragmented red blood cells and schistocytes. In view of hemolysis and thrombocytopenia accompanied by oliguric renal failure with a possibility of TTP-HUS, he was started on therapeutic plasma exchange (TPE). In total, 1.5 plasma volumes were exchanged on three consecutive days with fresh frozen plasma and albumin as the replacement fluid. His hemoglobin and platelet count stabilized after the third session of TPE. His renal parameters and urine output also showed recovery after third TPE and dialysis was withheld [Figure 2]. The patient was discharged on day 14 with stable hemoglobin and platelet count and LDH in declining trend (724 U/L). The patient had a recurrent attack of pancreatitis 4 months later but did not have any other organ involvement this time. He underwent endoscopic ultrasound (EUS), which showed early changes of chronic pancreatitis [Figure 3]. On his follow-up 2 weeks post discharge, he had urine protein of 2+ and 24-hour urinary protein of 3.3 gram/day. Repeat evaluation done at 4 weeks showed 24-hour urinary protein 1.0 g/day and had further decreased to 200 mg/dl done 8 months post discharge. In view of settling proteinuria, biopsy was not done. Genetic workup comprising CFH, CFHR1, CFHR3 genes, and anti-factor H antibody were negative. Deficiency of a disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13) could not be assessed in our patient due to non-availability of the test. Patient is now 1 year since onset of his illness. He is currently fine and is being treated with antioxidants and pancreatic supplementation and maintains normal kidney function and blood counts.
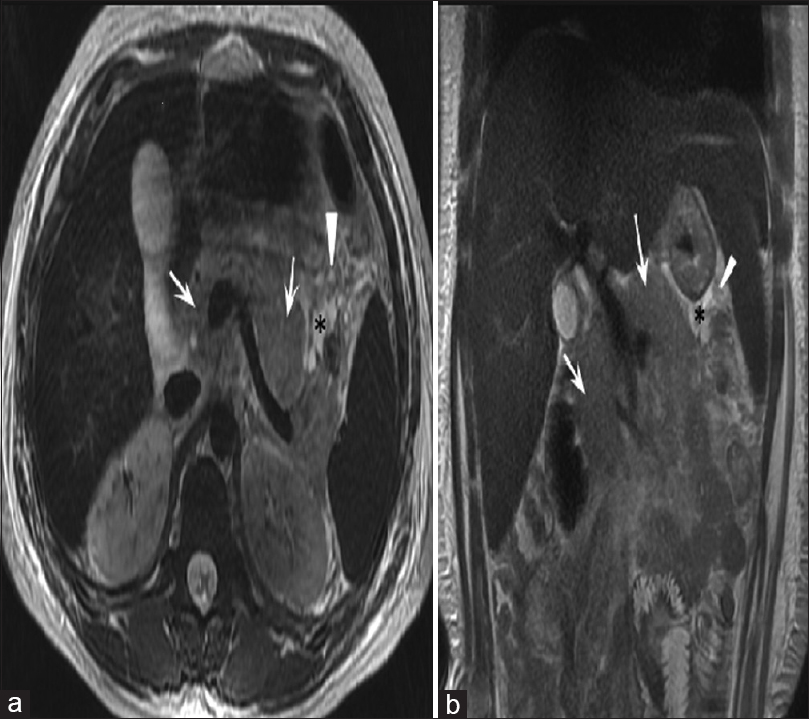
- MRI axial (a) and coronal (b) T2-weighted images of upper abdomen in a case of acute interstitial pancreatitis showing diffusely bulky pancreas (white arrows). There is also mild peripancreatic fluid (black asterisks) and fat edema (black arrows)

- Biochemical and hematological progression during admission and follow-ups
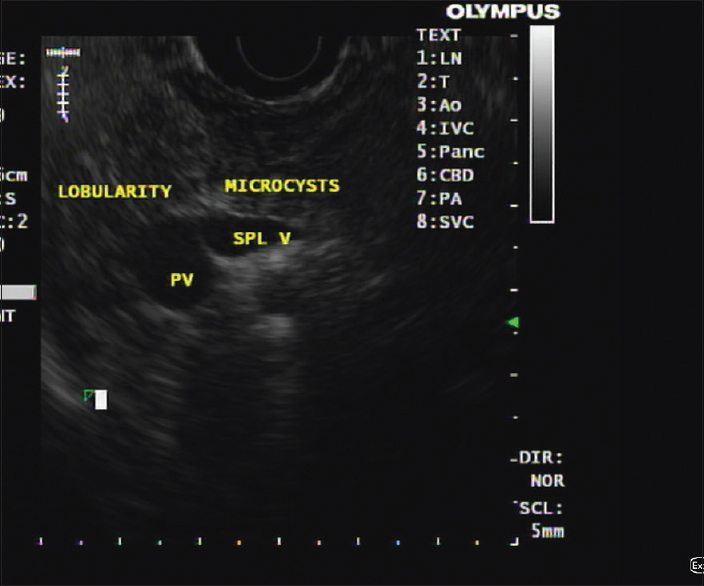
- Endoscopic ultrasound image showing lobularity of pancreatic parenchyma and microcysts indicative of early changes consistent with chronic pancreatitis
Discussion
TMA is an uncommonly reported complication of acute pancreatitis. Pancreatitis is seen in 2% of cases of TMA, but pancreatitis causing TMA is even less reported. The temporal association between pancreatitis and TMA is unclear, but it is hypothesized that cytokines such as interleukin-1 and tumor necrosis factor-α are released during pancreatitis, which cause vascular endothelial damage.[5] ADAMTS13, a plasma protease complex, prevents platelet aggregation by cleaving von Willebrand factor. In acute pancreatitis-related TMA, ADAMTS13 is inhibited by cytokines produced by ongoing inflammation, resulting in platelet aggregation and thrombus formation.[6]
Our case presented with pancreatitis with no other organ involvement to start with but was followed by development of bicytopenia (hemolytic anemia + thrombocytopenia) and renal failure on day 3 of his illness. Consistent with his presentation, TMA was considered. Considering the high mortality rate of untreated TMA and the benefits of early therapy, we initiated the patient on TPE. A similar line of treatment was offered to patients in the other reported cases.[78] In our patient, TMA happened on the third day of acute pancreatitis, similar to what has been described in the literature, although it may present as late as 2 weeks in some cases.[578] Hematological response defined as normalization of the platelet count without ongoing hemolysis on two occasions was achieved in our patient after third TPE.[2] In view of improvement post TPE, a diagnosis of secondary TMA secondary to acute pancreatitis was established in our case. ADAMTS 13 levels were not done in our patient due to the non-availability of the test. TMA manifests if ADAMTS 13 enzyme is severely deficient (<10%) but may be normal or falsely low in pancreatitis-related TMA even when autoantibodies are present.[9] Stool culture, complement levels, and complement factors (CFH, CFHR1, and CFHR3 genes), autoantibodies (anti-factor H), porphyria screening, and drug screening were all negative in our case.
Limitations of the case
The exact etiology of acute pancreatitis could not be proven in spite of exhaustive workup. Genetic workup for pancreatitis could not be done. The diagnosis of TMA was established based on the temporal association of events and based on the improvement shown after TPE. Renal biopsy was difficult at the time of admission and then in view of resolving proteinuria and normal renal function, it was decided against a renal biopsy so a tissue diagnosis of TMA could not be established. ADAMTS 13 levels were not available to comment upon.
Conclusions
TMA occurrence post-acute pancreatitis is uncommon but well established. Early recognition and management of this catastrophic complication can significantly affect overall morbidity and mortality.
Informed consent
Informed consent was obtained from the patient's parent/guardian.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Thrombotic microangiopathy care pathway: A consensus statement for the Mayo clinic complement alternative pathway thrombotic microangiopathy (CAP-TMA) Disease-oriented Group. Mayo Clin Proc. 2016;91:1189-211.
- [Google Scholar]
- Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15:312-22.
- [Google Scholar]
- How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood. 2010;116:4060-9.
- [Google Scholar]
- Thrombotic microangiopathy: An atypical cause of acute renal failure in patients with acute pancreatitis. Intensive Care Med. 2004;30:1235-9.
- [Google Scholar]
- Pancreatitis preceding acute episodes of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: Report of five patients with a systematic review of published reports. Haematologica. 2007;92:936-43.
- [Google Scholar]
- TTP and ADAMTS13: When is testing appropriate? Hematology Am Soc Hematol Educ Program. 2007;2007:121-6.
- [Google Scholar]
- A rare case of thrombotic microangiopathy triggered by acute pancreatitis. BMJ Case Rep 2017 bcr2016218581
- [Google Scholar]
- Thrombotic microangiopathy as a complication of recurrent pancreatitis. Indian J Nephrol. 2011;21:215-7.
- [Google Scholar]
- Lessons from acute pancreatitis-induced thrombotic thrombocytopenic purpura. Eur J Intern Med. 2009;20:739-43.
- [Google Scholar]







