Translate this page into:
The Frequency, Causes and Patterns of Asymptomatic Cardiac Arrhythmias in Patients on Maintenance Hemodialysis
Corresponding author: Deepak Phalgune, Department of Research, P D Hinduja National Hospital and Medical Research Centre, Mahim, Mumbai, India. E-mail: dphalgune@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sakhare Y, Almeida A, Phalgune D, Erande A, Mehendale SM. The Frequency, Causes and Patterns of Asymptomatic Cardiac Arrhythmias in Patients on Maintenance Hemodialysis. Indian J Nephrol. 2025;35:397-401. doi: 10.25259/ijn_412_23
Abstract
Background
The knowledge of the incidence of non-severe and clinically significant arrhythmias is limited in patients with chronic kidney disease (CKD). The present study was conducted to determine the incidence, pattern and identify the factors predisposing to cardiac arrhythmias in patients on maintenance hemodialysis.
Materials and Methods
Forty-five patients were included in this prospective observational study conducted between June 2020 and November 2021. Patients ≥ 18 years of age on maintenance hemodialysis (three times/week for at least three months), with no intercurrent illness, uremic symptoms, and not hospitalized in the previous 3 months were included. Demographic and clinical characteristics of the patients were noted. Arrhythmias were recorded by attaching the Holter machine to all study patients undergoing hemodialysis. We estimated the incidence, identified the pattern of cardiac arrhythmias, and explored the factors predisposing to cardiac arrhythmias.
Results
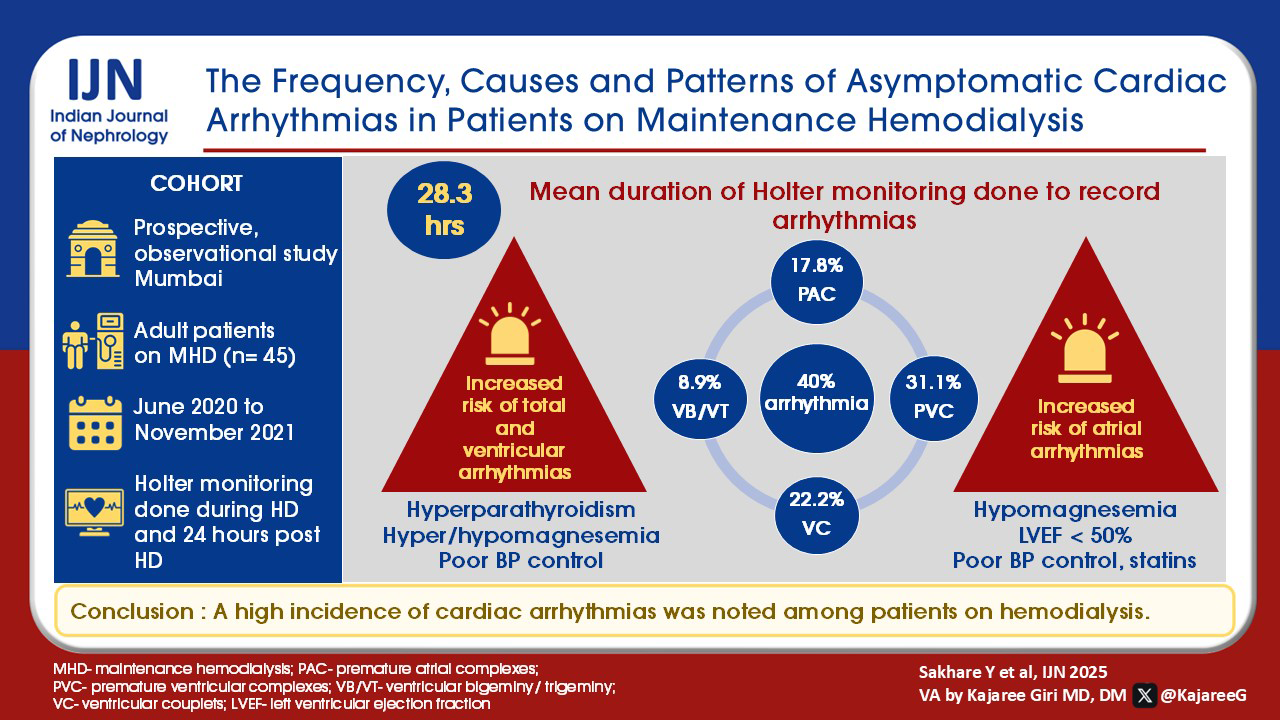
Premature atrial complexes (17.8%), premature ventricular complexes (31.1%), ventricular bigeminy (8.9%), trigeminy (8.9%), and ventricular couplets (22.2%) were observed. The patients with hyperparathyroidism, hyper/hypomagnesemia, and poor blood pressure control had significantly higher percentages of total and ventricular arrhythmias. The patients with hypomagnesemia, reduced left ventricular ejection fraction (<50%), poor blood pressure control, and receiving statins had significantly higher percentages of atrial arrhythmias. There was no statistically significant association between age, gender, diabetes mellitus, ischaemic heart disease, interdialytic weight gain, dialysis vintage, low hemoglobin, serum calcium levels, serum potassium levels, presence of left ventricular hypertrophy, pulmonary hypertension, and diastolic dysfunction with arrhythmias.
Conclusion
A high incidence of cardiac arrhythmias was noted among patients on hemodialysis.
Keywords
Cardiac arrhythmias
Chronic kidney disease
Hemodialysis
Introduction
Chronic kidney disease (CKD) is a leading cause of morbidity and mortality worldwide. Cardiovascular diseases (CVDs) remain the primary leading cause of death in patients on maintenance hemodialysis (HD), accounting for nearly 41% of deaths. Almost half of them are attributed to sudden cardiac death (SCD).1 Within this complex disease comorbidity, a large proportion of cardiovascular events have been reported to be related to the development of fatal cardiac arrhythmias.2,3 Mortality events are high on the days when HD is performed. This observation suggests that cardio-vascular events are associated with the procedure of HD itself.4 It is notable that up to one-third of outpatient HD treatments are complicated by episodes of intradialytic hypotension (IDH). Prior reports have noted an association between IDH and adverse events such as myocardial hypo-perfusion and stunning, which may predispose to the occurrence of cardiac arrhythmias.5,6 It is important to determine if such an association exists because IDH represents a potentially modifiable risk.7
Although the importance of arrhythmias in managing dialysis patients is well-recognized, the available evidence on the prevalence of both non-severe and clinically significant arrhythmias is limited.8 Previous studies have primarily been conducted in relatively small patient groups, possibly explaining the considerable variation in the reported prevalence of arrhythmias in patients receiving maintenance dialysis. Most of the data were obtained from short-term recordings with Holter monitoring before several significant advances in treating CVDs. Characteristically, the population undergoing dialysis has markedly changed since the earlier studies, and today, the patients are more likely to be older and have other comorbid conditions, including diabetes mellitus (DM).7 A change in the prevalence of arrhythmias is therefore anticipated. A registry study documented a marked increase in the prevalence of atrial fibrillation (AF) in patients receiving HD.9 On the other hand, since buffering is more frequently done with bicarbonate instead of acetate (acetate buffer is known to cause hypotension), the arrhythmogenic potential of an HD session may have been reduced during the last decades.10 Also, low-potassium dialysate (<2.0 mmol/L), which increases the risk of arrhythmias and SCD, was more often used previously.11,12 Various clinical characteristics independently associated with arrhythmias include older age, longer dialysis vintage, lower systolic blood pressure (SBP), history of palpitations, lower pre-dialysis plasma ionized calcium levels, and the presence of DM.8 Few studies are reporting the incidence of cardiac arrhythmias in Indian patients with CKD on maintenance HD. The present study was carried out to estimate the incidence, describe the pattern, and determine the factors that might predispose to cardiac arrhythmias in patients with CKD on maintenance HD.
Materials and Methods
This prospective observational study was conducted between June 2020 and November 2021 in the nephrology outpatient services of a tertiary care hospital in Mumbai, India, after obtaining approval from the institutional ethics committee (Letter No: IRB/1333/AL/20/17). Written informed consent was obtained from all the patients before enrolment after explaining the purpose of the study. Patients of stable CKD (no intercurrent illness or uremic symptoms, and not hospitalized in the previous 3 months) on maintenance HD (three times/week for at least three months), aged 18 or above, and with no cardiac arrhythmias at study entry assessment, were enrolled. Patients with known cardiac arrhythmias and on treatment were excluded.
The primary objectives were to estimate the incidence and describe the patterns of cardiac arrhythmia, whereas the secondary objective was to determine the factors predisposing to cardiac arrhythmias. The sample size was calculated by a formula N13 = (Zα)2p(1 – p)/d2 where Zα is a standard normal variate (at 5% type 1 error (p-value <0.05) = 1.96, p = expected proportion in population based on previous or pilot study and d is the absolute error or precision to be decided by researcher (= 0.05). Assuming the incidence of arrhythmias of 3%, a sample size of 45 patients was calculated.
Data was collected using a pre-tested study proforma. The proforma captured demographic and clinical characteristics and details of HD, such as duration, dry weight, interdialytic weight gain (IDWG), and IDH. The baseline investigations included serum potassium, calcium, magnesium, parathyroid hormone level, complete blood count, electrocardiogram, and 2D ECHO (Vivid E95, GE Healthcare 2 D ECHO machine). Holter findings were noted. The study was conducted on the 1st hemodialysis session of the week for all the patients.
All the patients underwent HD on B Braun Dialog Plus machines using a standard technique. The dialysate contained Part I, potassium 2.2 mmol/L, magnesium 0.50 mmol/L, calcium 1.50 mmol/L, sodium 138.0 mmol/L, chloride 109.0 mmol/L, and purified water. Part II contained sodium bicarbonate 59.20 gms/L and sodium chloride 23.50 gms/L.
Holter monitor (SCHILLER Medilog FD12 plus digital Holter recorder) was attached to patients undergoing HD and was kept attached for 24 hours post-dialysis. The data was recorded, and reports were analyzed for arrhythmias. For analysis, arrhythmias were categorized into bradyarrhythmias, tachyarrhythmias, and heart blocks.
The following time intervals were used for data analysis: P0: period on dialysis on the study day; P1: 0-6 hours post-dialysis period; P2: >6-24 hours post-dialysis. The incidence of arrhythmias was determined in these groups, and the patterns of arrhythmias were recorded.
IDWG was defined as an average interdialytic weight gain over the preceding last 4- weeks. The IDWG on the study day was defined as weight gain. The IDH was defined as a decrease in SBP ≥ 20 mm Hg or mean arterial pressure ≥10 mm Hg with associated symptoms.14 Poor blood pressure (BP) control was defined as average pre-dialysis BP ≥ 140/80 despite taking ≥2 antihypertensive drugs.15
Statistical analysis
The data collected were entered in Excel 2007, and analysis was done using the Statistical Package for Social Sciences for Windows, Version 26.0 from IBM Corporation, Chicago, IL, USA. The continuous data are presented as mean and standard deviation (SD), whereas the discrete data are presented as numbers and percentages. Comparison between two categorical variables was performed using Fisher’s exact test. A p-value less than 0.05 was considered to be statistically significant.
Results
The demographic and clinical profile of the study population is presented in Table 1. The mean duration of Holter monitoring for patients enrolled was 28.3 hours. Arrhythmia (ECG changes) were not detected in any patient before initiation of dialysis on the study day. Echocardiographic findings are presented in Table 2. Echocardiography identified regional wall motion abnormality (RWMA), LVH, PAH, LVEF (<50%), and DD in 12 (26.7%), 32 (71.1%), 23 (51.1%), 10 (22.2%) and 41 (91.1%) patients respectively [Table 2].
| Variables | n (%) |
|---|---|
| Mean age in years ± SD | 51.2 ± 14.0 |
| Gender | |
| Male | 30 (66.7) |
| Female | 15 (33.3) |
| Comorbidities | |
| Diabetes mellitus | 21 (46.7) |
| Hypertension | 39 (86.7) |
| Ischaemic heart disease | 11 (24.4) |
| Mean systolic blood pressure (mm Hg) ± SD | 133.8 ± 14.7 |
| Mean diastolic blood pressure (mm Hg) ± SD | 79.6 ± 5.2 |
| Mean dialysis Vintage (months) ± SD | 39.4 ± 25.3 |
| Mean IDWG (Kg) ± SD | 2.3 ± 0.9 |
| Mean IDWG on study day (Kg) ± SD | 2.7 ± 1.1 |
| Cardiovascular medications | |
| Beta blockers | 21 (46.7) |
| Antiplatelet agents | 20 (44.4) |
| Statins | 18 (40.0) |
SD: Standard deviation, IDWG: Interdialytic weight gain
| Parameter | Grades | n (%) |
|---|---|---|
| RWMA | Present | 12 (26.7) |
| Left ventricular hypertrophy | No | 13 (28.9) |
| I | 20 (44.4) | |
| II | 10 (22.2) | |
| III | 2 (4.5) | |
| Left ventricular ejection fraction | <50% | 10 (22.2) |
| ≥50% | 35 (77.8) | |
| Pulmonary hypertension | No | 22 (48.9) |
| I | 14 (31.1) | |
| II | 4 (8.9) | |
| III | 5 (11.1) | |
| Diastolic dysfunction | No | 4 (8.9) |
| I | 29 (64.4) | |
| II | 10 (22.2) | |
| III | 2 (4.5) |
RWMA: Regional wall motion abnormality.
Out of the 45 patients studied, 18 (40%) were found to have arrhythmias. Atrial and ventricular arrhythmias were recorded in 8 (17.8%) and 17 (37.8%) patients respectively. A single patient had a sinus pause. Out of 8 patients who had atrial arrhythmias, 1 patient had AF. All the patients with atrial arrhythmias had PACs. Out of 17 patients who had ventricular arrhythmias, 14 (82.4%) had PVCs. No event of VT was documented in any patient.
Table 3 shows arrhythmias during dialysis (P0), 0-6 h post-dialysis (P1), and >6 to 24 h post-dialysis (P2). PAC, PVC, ventricular bigeminy, trigeminy, and ventricular couplets were present in 8 (17.8%), 14 (31.1%), 4 (8.9%), 4 (8.9%), and 10 (22.2%) patients, respectively [Table 3].
| Period of study | PAC (%) | PVC (%) | Bigeminy (%) | Trigeminy (%) | Ventricular couplets (%) |
|---|---|---|---|---|---|
| P0 | 7 (15.6) | 14 (31.1) | 4 (8.9) | 3 (6.7) | 5 (11.1) |
| P1 | 6 (13.3) | 13 (28.9) | 2 (4.4) | 3 (6.7) | 7 (15.6) |
| P2 | 6 (13.3) | 13 (28.9) | 2 (4.4) | 3 (6.7) | 4 (8.9) |
| Total | 8 (17.8) | 14 (31.1) | 4 (8.9) | 4 (8.9) | 10 (22.2) |
P0: dialysis period, P1: 0-6 hours post-dialysis, P2: >6-24 hours post-dialysis, PAC: Premature atrial complexes, PVC: Premature ventricular complexes.
The patients with hyperparathyroidism, hyper/hypomagnesemia, and poor blood pressure control had significantly higher percentages of total arrhythmias. The patients on statins, hypomagnesemia, LVEF (<50%), and poor blood pressure control had substantially higher percentages of atrial arrhythmias. Patients with hyperparathyroidism, hyper/hypomagnesemia, and poor blood pressure control had a significantly higher percentage of ventricular arrhythmias [Table 4]. Though 21 patients were on different beta blockers (metoprolol-7, carvedilol-12, propranolol-2), no significant association was found between arrhythmias and beta-blocker use (p = 0.14). No statistically significant association existed between other factors like age, gender, DM presence, ischaemic heart disease (IHD), IDWG, dialysis vintage, hemoglobin %, calcium, potassium, LVH, PAH, and DD with arrhythmias.
| Variable | Atrial Arrhythmias | Ventricular Arrhythmias | Total Arrhythmias | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Absent | Present | p-value | Absent | Present | p-value | Absent | Present | p-value | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
| Statins | |||||||||
| No | 25 (92.6) | 2 (7.4) | 0.0447 | ||||||
| Yes | 12 (66.7) | 6 (33.3) | |||||||
| Magnesium levels | |||||||||
| Hyper | 14 (82.4) | 3 (17.6) | 0.0069 | 7 (41.2) | 10 (58.8) | 0.0058 | 7 (41.2) | 10 (58.8) | 0.0135 |
| Hypo | 0 (0.0) | 2 (100.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | 2 (100.0) | |||
| Normal | 23 (88.5) | 3 (11.5) | 21 (80.8) | 5 (19.2) | 20 (76.9) | 6 (23.1) | |||
| LVEF | |||||||||
| <50% | 6 (60.0) | 4 (40.0) | 0.0393 | ||||||
| ≥50% | 31 (88.6) | 4 (11.4) | |||||||
| Blood pressure | |||||||||
| Poor control | 1 (33.3) | 2 (66.7) | 0.0234 | 0 (0.0) | 3 (100.0) | 0.0229 | 0 (0.0) | 3 (100.0) | 0.0299 |
| Controlled | 36 (85.7) | 6 (14.3) | 28 (66.7) | 14 (33.3) | 27 (64.3) | 15 (35.7) | |||
| Parathormone | |||||||||
| High | 4 (33.3) | 8 (66.7) | 0.0172 | 4 (33.3) | 8 (66.7) | 0.0295 | |||
| Normal | 24 (72.7) | 9 (27.3) | 23 (69.7) | 10 (30.3) | |||||
Fisher’s exact test was used. LVEF: Left ventricular ejection fraction.
Discussion
Patients with CKD on maintenance dialysis experience a high rate of mortality, partly due to SCD caused by arrhythmias. The prevalence of arrhythmias, including its clinically significant subsets, is probably because the definitions differ. The present study sought to estimate the incidence of arrhythmias, characterize the arrhythmia pattern, and identify associated risk factors in HD patients.
Mahamood et al. reported PAC in 40.3% and PVC in 59.7% of patients.5 Rantanen et al. noted PAC in 100% and PVCs in 87.8% of their study population, and PACs were equally distributed on dialysis and non-dialysis days.9 Bigeminy at 20.3%, trigeminy at 22.0%, and couplets at 36.6% were observed in the study population.9 Narula et al. reported a prevalence of ventricular arrhythmias in 29.0% and supraventricular arrhythmias in 52.6%.16 Saygi et al. observed PVC in 68% of patients.17 Burton et al. detected PVCs in 63% of patients, and the total frequency of PVCs was higher during HD than in the post-dialysis phase (0.26% vs 0.12%, p < 0.01).18 In 221 outpatients receiving HD, the occurrence of PVCs (11.8%), supraventricular premature contractions (6.8%), first-degree AV block (5.4%), AF (5.4%), complete right bundle branch block (3.2%), incomplete right bundle branch block (1.8%), sinoatrial block (0.4%), bi-fascicular block (0.4%) was reported.19 Ramirez et al. stated that 12/30 (40.0%) patients had arrhythmias (2 had VT, 2 had AF).20 Genovesi et al. noted AF in 27.0% of patients [paroxysmal (3.5%), persistent (9.6%), permanent (13.9%)].21 Vazquez et al., reporting on 256 patients studied, found 31(12.1%) had AF at the start of dialysis.22 Using implantable loop recorders, the prevalence of non-sustained VT varied between 14 and 57%.23,24 The absence of heart blocks in the present study population could have resulted from monitoring for a shorter duration.
Saygi et al. concluded that patients with PVC were significantly older, with a longer HD duration and higher hemoglobin levels.17 Burton et al. noted no statistically significant differences in the frequency of PVCs with age, gender, dialysis vintage, DM, hypertension, and a beta-blocker (p > 0.05). However, patients with pre-existing IHD had an increased frequency of PVCs during HD (1.19% vs. 0.17%, p < 0.03), as also noted in patients with LVH (0.87% vs. 0.12%, p < 0.02).18 Patients with very high serum parathormone levels and those with valvular disease, low LVEF, LVH, or left atrial enlargement on echocardiography appeared to be at risk for developing intradialytic atrial arrhythmias.25 Abbot et al. reported that the factors associated with AF were older age (≥71 years vs. <48 years), extremes (both high and low) of pre-dialysis SBP, and digoxin use.26 In another study, age, gender, DM, smoker status, diastolic blood pressure (DBP), hematocrit, serum creatinine, and antihypertensive medication were not associated with arrhythmia.27 Roy-Chaudhary et al., reporting higher age association with clinically significant arrhythmias, suggested the potential for future dialysis process of care interventions to reduce mortality.23 Ramirez et al. pointed out arrhythmia was associated with high parathormone levels but with no statistically significant difference with age, duration of dialysis, weight gain during dialysis, hemoglobin, creatinine, calcium, sodium, potassium, chloride, bicarbonate, blood urea nitrogen, phosphate, 2D echocardiography findings, systolic blood pressure, DBP between patients who developed and didn’t develop arrhythmias.20 Narula et al. stated that there was no difference in the age, sex ratio, duration of HD, BP, fluctuations in weight, hematocrit, pre-dialysis creatinine, sodium, potassium, calcium, or inorganic phosphate levels between patients with or without ventricular arrhythmias. The study further stated that the number of patients with clinical IHD was significantly greater in the ventricular arrhythmias group. In contrast, patients with ventricular arrhythmias had a higher proportion of patients with LVH and left ventricular dysfunction, but the difference failed to reach statistical significance.16 Genovesi et al. noted that patients with AF were older (71.8 vs 64.7, p < 0.01), and its prevalence increased with age.21 Also, AF was associated significantly with IHD, dilated cardiomyopathy, acute pulmonary edema, valvular disease, cerebrovascular accidents, and predialysis hyperkalemia.21 Patients with AF more frequently showed left atrial dilatation, and LVEF was significantly lower. No association was found between arrhythmia and hypertension or DM. Multivariate analysis confirmed that patient age, duration of HD therapy, and left atrial dilatation were associated with AF.21 Vazquez et al. reported that increased age, larger left atrium, and female gender were independently related to the presence of AF at dialysis inception.22
A limitation of our single-center study is its small sample size. Monitoring for 28 hours resulted in a point-in-time estimate of the prevalence of arrhythmias and might have resulted in an underestimation of the arrhythmia burden. We could not study the cause-and-effect relationship between arrhythmias and various study variables. We did not have data for post-dialysis serum potassium levels. The association between potassium level and adverse events is likely mediated by post-dialysis serum potassium level. External Holter devices might not be as sensitive as an implantable loop recorder, used in other studies. The results would have been more robust if we had compared the patients’ QT interval and arrhythmias (PAC and PVC) with a control group. Further investigation is needed to clarify the mechanisms underlying arrhythmias in patients receiving HD to improve the quality of life and prognosis.
As patients receiving maintenance dialysis have a high prevalence of both non-severe and clinically significant arrhythmias, we suggest that frequent monitoring of patients on hemodialysis might help in identifying asymptomatic arrhythmias and prevent severe catastrophes like cardioembolic stroke, myocardial ischemia, and sudden cardiac death. Several variables were associated with arrhythmias, but further studies are warranted to increase the understanding of these associations and clarify the extent to which arrhythmias predict outcomes in this high-risk population.
Conflicts of interest
There are no conflicts of interest.
References
- Sudden cardiac death and acute myocardial infarction in dialysis patients: Perspectives of a cardiologist. Semin Nephrol. 2005;25:363-6.
- [CrossRef] [PubMed] [Google Scholar]
- Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473-82.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of cardiac arrhythmias in chronic renal failure, especially during hemodialysis. Nephron. 1991;57:500-1.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19-26.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013;3:19-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- US renal data system 2020 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2021;77:A7-A8.
- [CrossRef] [PubMed] [Google Scholar]
- Global dialysis perspective: India. Kidney 360.. 2020;1:1143-7.
- [CrossRef] [PubMed] [Google Scholar]
- Arrhythmias in patients on maintenance dialysis: A cross-sectional study. Am J Kidney Dis. 2020;75:214-24.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular disease in chronic kidney disease. A clinical update from kidney disease: Improving global outcomes (KDIGO) Kidney Int. 2011;80:572-86.
- [CrossRef] [PubMed] [Google Scholar]
- Severe coronary stenosis is an important factor for induction and lengthy persistence of ventricular arrhythmias during and after hemodialysis. Am J Kidney Dis. 2004;44:328-36.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121-6.
- [CrossRef] [PubMed] [Google Scholar]
- K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1-153.
- [PubMed] [Google Scholar]
- Hypertension in patients on dialysis: Diagnosis, mechanisms, and management. J Bras Nefrol. 2019;41:400-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cardiac arrhythmias and silent myocardial ischemia during hemodialysis. Ren Fail. 2000;22:355-68.
- [CrossRef] [PubMed] [Google Scholar]
- Ventricular arrhythmia in dialysis patients: A link with higher hemoglobin levels? Hemodial Int. 2011;15:250-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodialysis-induced left ventricular dysfunction is associated with an increase in ventricular arrhythmias. Ren Fail. 2008;30:701-9.
- [CrossRef] [PubMed] [Google Scholar]
- Electrocardiographic abnormalities in patients receiving hemodialysis. Am Heart J. 1996;131:1137-44.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac arrhythmias on hemodialysis in chronic renal failure patients. Nephron. 1984;36:212-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis. 2005;46:897-902.
- [CrossRef] [PubMed] [Google Scholar]
- Atrial fibrillation in incident dialysis patients. Kidney Int. 2009;76:324-30.
- [CrossRef] [PubMed] [Google Scholar]
- Primary outcomes of the monitoring in dialysis study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int. 2018;93:941-51.
- [CrossRef] [PubMed] [Google Scholar]
- Original article predictors of arrhythmic events detected by implantable loop recorders in renal transplant candidates. Arq Bras Cardiol. 2015;105:493-502.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Symptomatic atrial arrhythmias in hemodialysis patients. Ren Fail. 2001;23:71-6.
- [CrossRef] [PubMed] [Google Scholar]
- Atrial fibrillation in chronic dialysis patients in the United States: Risk factors for hospitalization and mortality. BMC Nephrol. 2003;24(4):1.
- [PubMed] [Google Scholar]
- Blood pressure and the risk of complex arrhythmia in renal insufficiency, hemodialysis, and renal transplant patients. Am J Hypertens. 1999;12:204-8.
- [CrossRef] [PubMed] [Google Scholar]








