Translate this page into:
The Gamma Gap Predicts All-Cause Mortality in Chronic Dialysis Patients
Address for correspondence: Prof. Petar J. Avramovski, Ivan Milutinovik No. 37/4-26, 7000 Bitola, North Macedonia. E-mail: avramovski@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The gamma gap (γ-gap) represents the total serum protein concentration minus the albumin concentration. The main aim of this study was to test whether the gamma gap is a predictor of mortality and whether it is associated with other predictors of mortality in chronic hemodialysis patients (CHPs).
Materials and Methods:
We studied a cohort of 100 CHPs with a mean age of 59 ± 12.3 years with duration of dialysis 6.5 ± 4.7 years. Serum proteins were determined by electrophoresis. The association of the gamma gap with serum C-reactive protein (CRP), fibrinogen and albumin concentration was evaluated for correlation. Cox regression analysis was used to identify the predictors of mortality.
Results:
The γ-gap correlates positively with CRP (r = 0.247, P = 0.013) and fibrinogen (r = 0.239, P = 0.016), and inversely with albumin (r = -0.430, P < 0.0001). The regression coefficients (b) and Exp (b) hazard ratio coefficients of covariates in Cox-regression survival analysis in all-cause outcomes were: b = 0.1486, Exp (b) = 1.1602 (P < 0.0001); b = 0.0655, Exp (b) = 1.0677 (P < 0.0015) and b = -0.118, Exp (b) = 0.8887 (P < 0.0009), for γ-gap, CRP and albumin, respectively.
Conclusions:
In patients on chronic hemodialysis, the gamma gap, along with serum albumin and CRP levels, is an independent predictor of mortality. Gamma gap levels correlate directly with serum CRP and fibrinogen levels and inversely with serum albumin levels.
Keywords
Albumin
dialysis
C-reactive protein
gamma gap
mortality predictors
Introduction
The gamma gap (γ-gap), alternatively known as the paraprotein gap, represents the total serum protein concentration minus the albumin concentration, presented by equation 1: (γ-gap = total protein-albumin). An elevated γ-gap is usually considered to be an indicator of latent infection, systemic inflammation, autoimmune inflammatory disease or malignancy.[12] An arbitrary value of 40 g/L is considered a positive γ-gap even though there are not enough prospective studies examining γ-gap in association with clinical outcomes.[34] Serum proteins albumin, C-reactive protein (CRP), interleukin 6, carrier proteins, complement and immunoglobulins are useful markers of inflammation.[5] Chronic low-grade inflammation plays a key role in the process of aging, arterial stiffening and increased mortality risk.[67] Between 30 and 50% of CHPs have modified levels of serum proteins as inflammatory markers.[89] The main aim of this study was to test whether the gamma gap is a predictor of mortality and whether it is associated with other predictors of mortality in CHPs.
Materials and Methods
This study was designed as a prospective cohort analysis in 100 CHPs who underwent clinical laboratory procedures at baseline and every next 6 months with a 48-month follow-up period. Demographic and clinical data were collected from the patient's chart and included age, sex, weight, height, history of diabetes mellitus, smoking habit, hypertension, cardiovascular history including myocardial infarction and stroke, peripheral vascular disease, vascular surgery, revascularization, heart failure, and malignancy. The CHPs were eligible for entry into the study if they had been on chronic hemodialysis for at least 3 months and if they had no acute clinical manifestation of cardiovascular disease (stroke, myocardial infarction, peripheral vascular occlusion, heart failure) and malignancy at least 6 months before entering in the study. All participants signed informed consent and the ethics committee of our institution approved the study.
The hemodialysis session was tailored (4–5 h, three times a week) to achieve the average Kt/V at least 1.2 [Kt/V= 1.2 (1.255 ± 0.336)] with urea reduction rate (URR) = 65.18 ± 5.73%, using a low-flux synthetic membrane, at a blood flow rate of 185–210 mL/min via their arteriovenous fistulas. A bicarbonate dialysate was used at a flow rate of 500 mL/min in each patient. Clinical and biochemical parameters (total protein, albumin, α-1 globulin, α-2 globulin, β-globulin, γ-globulin, CRP, and fibrinogen) were determined in all participants using standard laboratory procedures, performed on a Cobas Mira S Analyzer (Roche Diagnostics, Holliston, MA) and electrophoresis performed on cellulose acetate system Compact SAIO (Elettronica Srl, Pompei [ISO 9001]) with electrophoresis KIT Aries. Blood was drawn immediately before the start of a dialysis session, early in the morning, in a fasting state. Mean values were obtained at baseline and every 6 months during a 48-month follow-up period.
Statistical analysis
Statistical analysis was performed using MedCalc Statistical Software version 18.2.1 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2018). The results were expressed as means ± SD or percentage, unless otherwise stated. Receiver operating characteristics (ROC) curve analysis assessed the distinction between patients who survived and patients who died from the all-cause event, and their survival rates were analyzed using Kaplan-Meier survival curves. The Cox proportional hazards regression model (backward stepwise) was used to identify the independent determinants of mortality predictors.
Results
Characteristics of participants
From March 2013 to March 2017, demographic data were collected from patients' medical charts and laboratory examinations were successfully conducted on 100 CHPs with a mean follow-up of 37.3 ± 15.2 months [9 to 48 months (10–90 percentiles)]. The patient's demographic and clinical characteristics calculated as the average value of multiple successive laboratory examinations are shown in Table 1. The mean, median, 25th and 75th percentiles, range and 95% confidence interval for the mean of the γ-gap and the albumin are shown with box and whisker plots in Figure 1. The outside values of γ-gap and albumin are presented with small blue circles and small orange squares, respectively.
| Variables | Mean, SD, n (%) | Median | Range |
|---|---|---|---|
| Demographic characteristics | |||
| Gender, male, n (%) | 62 (62) | / | / |
| Age, years | 59±12.3 | 56 | 37.5-78 |
| BMI, kg/m2 | 23.46±3.57 | 23.1 | 17-35.4 |
| Hypertension | 32 (32) | / | / |
| Diabetes | 18 (18) | / | / |
| Smokers | 23 (23) | / | / |
| Duration of dialysis, years | 6.5±4.7 | 4.25 | 0.3-24 |
| Biochemistry | |||
| Total protein, g/L | 66.98±6.37 | 66.3 | 52-80 |
| Albumin, g/L | 37.71±5.12 | 38 | 27.2-49.9 |
| α-1 globulin, g/dL | 0.561±0.1238 | 0.5 | 0.4-0.8 |
| α-2 globulin, g/dL | 0.897±0.2148 | 0.8 | 0.6-1.3 |
| β-globulin, g/dL | 1.07±0.1927 | 1 | 0.8-1.5 |
| γ-globulin, g/dL | 2.35±0.5383 | 2.2 | 1.6-3.7 |
| γ-GAP, g/L | 29.29±6.66 | 28.85 | 16-48 |
| CRP, mg/L | 11.42±7.67 | 8.83 | 0.21-47 |
| Fibrinogen, g/L | 4.21±1.18 | 3.8 | 2.6-7.6 |
The results are expressed as mean, standard deviation (SD), number (n), percent (%), median and range. CHPs: Chronic hemodialysis patients; BMI: Body mass index; CRP: C-reactive protein

- Box and whisker plots of the mean, median, 25th and 75th percentiles, range and 95% confidence interval for the mean of the γ-gap and the albumin
Patients' outcomes
Sudden events caused by cardiovascular and non-cardiovascular causes of deaths (all-cause mortality) were recorded in 41 CHPs (41%, 23 male and 18 female) during 48-month follow-up period and were marked by 1 (one). The results of the most common disease groups as the cause of death in 41 patients are shown in Table 2. There was statistically high significance between the mean γ-gap in the subgroup with endpoint 1 (41 non-survived CHPs, γ-gap = 32.85 ± 7.22) and subgroup with endpoint 0 (59 survived CHPs, γ-gap = 26.83 ± 4.96, P < 0.0001).
| List of causes of death | Number of patients (%) |
|---|---|
| Stroke | 5 (12.19) |
| Myocardial infarction | 6 (14.63) |
| Arrhythmia | 4 (9.75) |
| Pulmonary edema | 3 (7.31) |
| Sudden cardiac death | 4 (9.75) |
| Malignant tumors | 4 (9.75) |
| Non-ketotic hyperglycemic syndrome with coma | 2 (4.87) |
| Ketoacidosis | 2 (4.87) |
| Infections | 3 (7.31) |
| Gastrointestinal bleeding | 5 (12.19) |
| Other causes | 3 (7.31) |
| Total 41 non-survival | n=41 (99.93%) |
CHPs: Chronic hemodialysis patients
Cutoff point estimation
Mostly based on ROC analysis, there are various methods to determine the test cutoff value.[10] The summary image of two ROC curves for γ-gap and albumin as a prognostic marker for the all-cause event are shown in Figure 2.
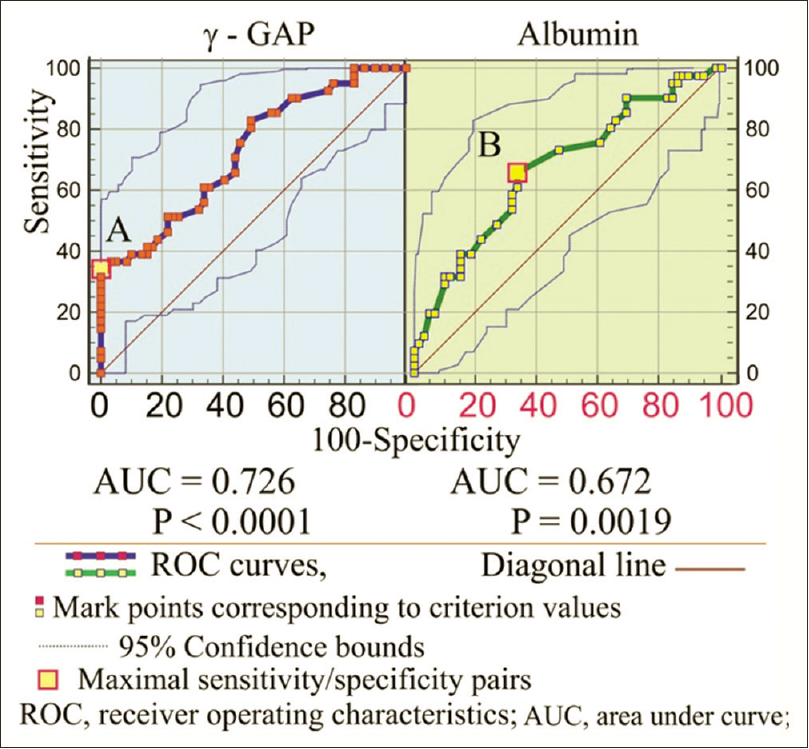
- Receiver-operating characteristics curves for γ-gap and albumin as prognostic markers for all-cause event
Survival and Cox regression analysis
During the follow-up period, 41 deaths were recorded. The mean survival time was 37.3 ± 1.52 months (CI = 34.304 to 40.316). We inserted in the Cox model the following covariates: total protein, albumin, α-1 globulin, β-2 globulin, γ-globulin, CRP, fibrinogen, γ-gap, dialysis duration, BMI, hypertension, diabetes and smoking status. Because of its multicollinearity phenomenon (strong inter-correlation of age with albumin, CRP, γ-globulin and hypertension) we did not enter an age in Cox-regression analysis. According to the Cox-regression analysis, the significant covariates retained by the model (backward stepwise) were only γ-gap, albumin, and CRP.
The predictor variables γ-gap and CRP with positive regression coefficient (b = 0.1486 and b = 0.0655, respectively) were associated with increased hazard and decreased survival times. Albumin with its negative regression coefficient (b = -0.118) was associated with increased hazard and decreased survival times, too, but in the inverse direction. Its hazard ratio (HR) coefficient Exp (b) = 0.8887, indicates that HR increased by 1.125239 (1/0.8887) with each unit decrease by self, or HR increased for 12.52% with each one g/L decrease of albumin (probability 99.9991%).
Assessments (regression coefficient [b], standard error [SE], Wald, P value, hazard ratio coefficient Exp [b], and 95% CI [confidence interval] of Exp [b]) of independent predictors for all-cause outcome after Cox-regression model analysis are shown in Table 3. A plot of the Kaplan–Meier estimate of the survival function presented as series of horizontal steps of declining magnitude, approaching the true survival function in CHPs is shown in Figure 3. Vertical drop indicates an event.
| Cox proportional-hazards regression | ||||||
|---|---|---|---|---|---|---|
| Cases summary | ||||||
| Number of eventsa | 41 | 41% | ||||
| Number censoredb | 59 | 59% | ||||
| Total number of cases | 100 | 100% | ||||
| aEnd point=1; bEnd point=0. | ||||||
| Method, backward. Enter variable if P<0.05; Remove variable if P>0.1 | ||||||
| Overall model fit | ||||||
| Null model -2 Log-likelihood | 342.507 | |||||
| Full model -2 Log-likelihood | 306.706 | |||||
| Chi-squared | 35.801 | |||||
| Significance level | P<0.0001 | |||||
| Coefficients and Standard Errors | ||||||
| Covariate | b | SE | Wald | P | Exp (b) | 95% CI of Exp (b) |
| α-gap | 0.1486 | 0.0290 | 26.2568 | <0.0001 | 1.1602 | 1.0964-1.2278 |
| Albumin | -0.118 | 0.0355 | 11.0486 | 0.0009 | 0.8887 | 0.8293-0.9524 |
| CRP | 0.0655 | 0.0206 | 10.1099 | 0.0015 | 1.0677 | 1.0256-1.1114 |
| Dialysis duration | 0.6078 | 0.3289 | 3.4138 | 0.0647 | 1.8363 | 0.9637-3.4990 |
Variables not included in the model: Total protein, α-1 globulin, α-2 globulin, β-globulin, α-globulin, fibrinogen, body mass index, hypertension, diabetes, and smoking status. b, b regression coefficient; P value of statistical significance; Exp [b]-hazard ratio coefficient. CI: Confidence interval; CRP: C-reactive protein; SE: Standard error
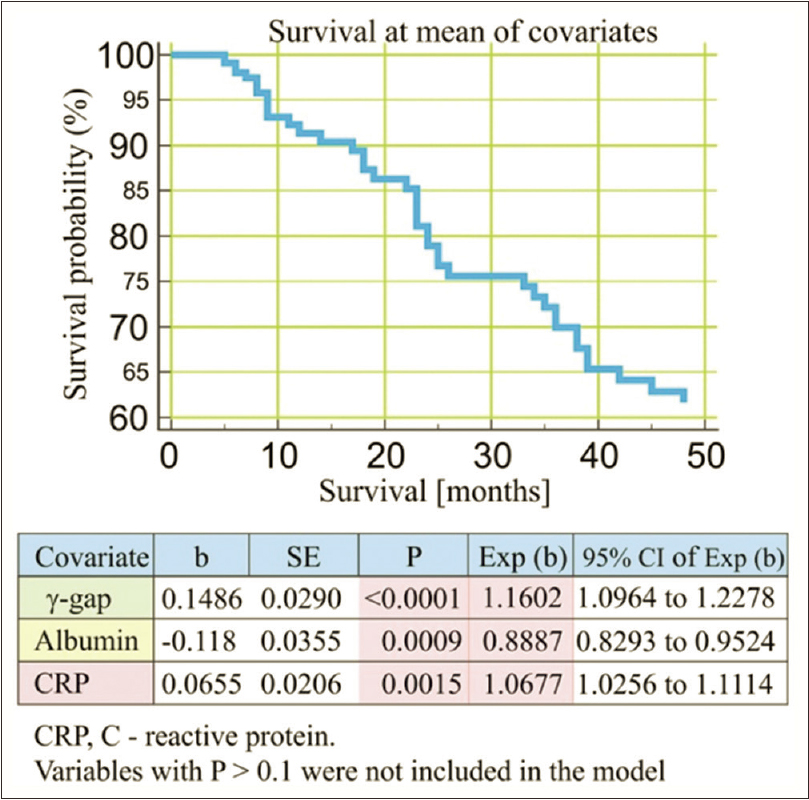
- Cox-regression survival analysis (predictors of all-cause outcome)
Discussion
An electronic search under the term “gamma gap and renal diseases” yielded zero references. After removing the quotes, search with the same term as a keyword showed only several studies that included participants from the general population[135] but not studies among CKD. The small number of obtained references in the field of the gamma gap and renal diseases was the motivation for this research in CHPs. Some of the studies investigated the gamma gap prediction in all-cause mortality in the older age population,[3511] but there are no studies that estimated the gamma prediction of all-cause mortality in CKD population.
Despite the widespread application of the γ-gap in clinical practice, there is currently very little literature guiding its use.[3] To our knowledge, this is the first longitudinal prospective study that evaluates all-cause mortality in CHPs and that identifies the serum proteins (total protein, albumin, α-1 globulin, α-2 globulin, β-globulin, γ-globulin), calculated γ-gap, CRP and fibrinogen as determinants of mortality predictors.
The results for γ-gap among non-survived patients in our study are in line with the results of recent cohort study by Juraschek et al.[3] who reported that an elevated γ-gap ≥31 g/L was an independent risk factor for all-cause mortality among a large population of U.S. adults.[3] Although in clinical practice, a somewhat arbitrary threshold of 35 g/L or 40.0 g/L is used, Loprinzi[5] used the cutoff point of 31.0 g/L in a study which examined the association between the γ-gap and all-cause mortality risk,[5] the same value as in ours and in the Juraschek's study. Because of the different values of the analyzed cutoff values for γ-gap in previously mentioned studies, we presented results for different likelihood ratios for γ-gap in intervals from 32 to 37 g/L with calculated risk for all-cause mortality 1.2 to 4.3 times increased than CHPs with the mean (29.29 ± 6.66 g/L) values of γ-gap.
The previously mentioned study by Juraschek et al.[3] evaluated different cut-off points that might be used to define a positive γ-gap.[3] They defined that γ-gap was associated with all-cause mortality when it is ≥29 g/L. It is the same value of γ-gap with the mean value for calculating the likelihood ratios in our study with an HR risk of 1.39.
Bearing in mind that we compared two different population groups (CHPs in our study and general population in other studies), it is expected that the results of the mean value of the γ-gap in our study (29.29 ± 6.66 g/L, in 100 CHPs) will be significantly different (P < 0.0001, t = 4.15) from the results of other studies (27.0 ± 5.0 g/L, 801 general population participants).[12] The significantly higher γ-gap value in CHPs is due to the reduced albumin level (according to equation 1) because of malnutrition and inflammation, acute-phase reactants and the plasma volume status accompanied by albumin redistribution, exogenous loss and decreased albumin synthesis.[13] The differences in γ-gap in both examined populations also result from higher total protein values in the general population compared to CHPs. Low values of total protein in CHPs are a result of the high prevalence of protein-energy malnutrition, increased protein catabolism due to acidemia, losses into dialysate of such biologically valuable nitrogenous compounds as amino acids, peptides, and proteins. Possible causes for hypoproteinemia include resistance to anabolic hormones (insulin, insulin-like growth factor-1) and a chronic inflammatory state associated with increased levels of pro-inflammatory cytokines.[1141516]
The associative connection of γ-gap with serum acute-phase proteins, however strong it is, does not mean that mentioned variables (CRP, fibrinogen, total protein, and albumin) would be strong predictors for all-cause mortality in CHPs. We found an important fact that γ-gap is not correlated with age (P = 0.741) and with dialysis duration (P = 0.472). In many studies, the strong correlation of age with other predictors of mortality is not favorable due to its statistical impact, called multicollinearity. In our study, there is no phenomenon of multicollinearity, which emphasizes the predictor importance of other variables, excluding the impact of aging and dialysis duration. In this way, the impact of γ-gap on the all-cause mortality is more realistic, because of the impact of the natural aging process which is reduced to a minimum. The lack of correlative relationships between these variables is an encouraging fact that mortality should not largely dependent on the natural aging process or dialysis. This assumption will be confirmed by the Cox-regression analysis.
The initial idea to show the γ-gap impact on cardiovascular mortality in CHPs (beside all-cause mortality) was rejected because of the lack of statistical significance (P = 0.518) between deceased patients who died from cardiovascular disease and patients registered that died from all-causes mortality. Comparing the clinical variables in the survived and non-survived CHPs as potential predictors for all-cause mortality, we noted reduced survival time in elderly patients, in patients with an elevated level of γ-gap, fibrinogen, CRP and total protein, and in patients with a decreased level of albumin. We kept our attention on the γ-gap and associated variables albumins, and CRP with their prognostic significance in determining all-cause mortality, presented by ROC curves and Cox regression analysis. The ROC curve results of γ-gap and albumin showed a close similar significant surface area and significant sensitivity and specificity of these variables in the detection of an all-cause lethal event.
As opposed to the traditional clinical practice of defining a γ-gap at a value of 40 g/L, we showed maximal likelihood ratio with an increased risk of mortality of 4.317 for γ-gap ≥37 g/L.[13] The increased risk for all-cause mortality in our study is still persistent and significant even at lower values for γ-gap (31–35 g/L) with a likelihood ratio of approx. 1.2 unlike the results of Juraschek et al.[3] who showed that γ-gap cutoff value above 37 g/L was non-significant. For these reasons, the γ-gap cutoff value of 37 g/L which is not statistically significant as a threshold value (survived/deceased) in the general population, in our study presents the highest sensitivity and specificity pair for a variable in the detection of all-cause mortality.
We also confirmed the prediction value of the γ-gap, albumin and CRP in the 4-year follow-up period. The γ-gap HR risk (1.16) for all-cause mortality increases by 16.02% with its each unit increase, albumin HR risk (-1.12) increases by 12.52% with its unit decrease and CRP HR risk (1.06) increases by 6.77% with its unit increase. Comparing the HR results of Juraschek's study[3] presented as the association between γ-gap and all-cause mortality at different cutoff points we concluded that our results are very close: 1.11 to 1.36 HRs for cardiovascular and all-cause mortality, for every unit increase of γ-gap. The similar and very close results were in Yang et al. (2018) study: HR of 1.19 for γ-gap as continuous variable per every unit increase.[12] We are aware of the inadequate comparison of the HR risk results of the γ-gap between two different populations (CHPs and the general population). We consider that the lack of studies among chronic renal disease patients justified this kind of comparison.
Because of this fact, the influence of inflammation on cardiovascular events must not be neglected, which in our study was 43.8% of the deceased CHPs. When compared with the general population, dialysis patients have been reported to have an up to a 100-fold increase in the age-adjusted risk of mortality due to infections associated with sepsis, as well as a 10-fold increased risk of death due to pulmonary infection.[1718] Inflammation is an important predictor of low serum albumin levels among dialysis patients, independent of nutritional status. Both serum CRP and albumin levels are predictors of all-cause mortality. Even in the absence of severe inflammation, the presence of severe hypoalbuminemia, regardless of CRP level, is associated with elevated in-hospital mortality.[1920] Several recent studies have confirmed that inflammation, as reflected by elevated levels of serum CRP or proinflammatory cytokines, are significant independent predictors of mortality in hemodialysis patients.[21222324]
Study limitations
The first limitation of our study is the relatively small number of patients studied. The second limitation is a relatively short period of follow-up. Recruiting CHPs in a sufficient number with prolongation of the monitoring period would have yielded surely better results.
Conclusions
We conclude that γ-gap is an independent predictor of overall mortality in patients undergoing dialysis, side-by-side to other independent predictors: CRP as a prototypical positive acute-phase protein and serum albumin as a negative acute-phase protein, over a relatively short time period. The continuous monitoring of total protein and albumin may be beneficial in the clinical setting and we highlight a potentially beneficial role in CHPs with elevated gamma gap in the prevention and treatment of inflammatory syndrome, together with CRP and other biochemical inflammatory markers.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Atherogenic index of plasma and the gamma gap: Consideration by physical activity. Int J Cardiol. 2016;222:946-8.
- [Google Scholar]
- Retrospective cohort study of 148 patients with polyclonal gammopathy. Mayo Clin Proc. 2001;76:476-87.
- [Google Scholar]
- The gamma gap and all-cause mortality risk: Consideration of physical activity. Int J Clin Pract. 2016;70:625-39.
- [Google Scholar]
- Chronic inflammation: Accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;72:1218-25.
- [Google Scholar]
- Ageing process and stiffening of arteries shown by increased pulse wave velocity. JSM Atheroscler. 2018;3:1040-6.
- [Google Scholar]
- Does inflammation affect outcomes in dialysis patients? Semin Dial. 2018;31:388-97.
- [Google Scholar]
- Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684-8.
- [Google Scholar]
- Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837-45.
- [Google Scholar]
- On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochemia Medica. 2016;26:297-307.
- [Google Scholar]
- The gamma gap predicts 4-year all-cause mortality among nonagenarians and centenarians. Sci Rep. 2018;8:1046.
- [Google Scholar]
- Protein metabolism in patients with chronic renal failure: Role of uremia and dialysis. Kidney Int. 2000;58:1-10.
- [Google Scholar]
- Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448-54.
- [Google Scholar]
- Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: A practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 1999;13:273-9.
- [Google Scholar]
- Inflammatory syndrome in patients on hemodialysis. J Amer Soc Nephrol. 2006;17(suppl 3):274-80.
- [Google Scholar]
- Mortality from infections and malignancies in patients treated with renal replacement therapy: Data from the ERA-EDTA registry. Nephrol Dial Transplant. 2015;30:1028-37.
- [Google Scholar]
- Prognostic value of serum albumin combined with serum C-reactive protein levels in older hospitalized patients: Continuing importance of serum albumin. Aging Clin Exp Res. 2006;18:307-11.
- [Google Scholar]
- C-reactive protein and other markers of inflammation in hemodialysis patients. Caspian J Intern Med. 2013;4:611-6.
- [Google Scholar]
- C-reactive protein as an outcome predictor for maintenance hemodialysis patients. Kidney Int. 1998;54:627-36.
- [Google Scholar]
- Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. Am J Kidney Dis. 2003;42:286-94.
- [Google Scholar]
- Specific proteins. In: McPherson RA, Pincus MR, eds. Henry's Clinical Diagnosis and Management by Laboratory Methods. Chap 19 (22nd ed). Philadelphia, PA: Elsevier Saunders; 2011.
- [Google Scholar]







