Translate this page into:
Time of Cold Ischemia and Delayed Graft Function in a Cohort of Renal Transplant Patients in a Reference Center
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
There are many factors involved in the delayed graft function of a renal graft, with prolonged cold ischemia time being one of the most relevant. The aim of this study is to evaluate the relationship between the time of cold ischemia and the delayed graft function, and acute rejection and graft loss at 1 year of follow-up. A retrospective cohort of 347 renal transplant patients were evaluated during the years 2009–2013. The incidence of delayed graft function was 18.4% (n = 65). The cold ischemia time was an independent risk factor for delayed graft function (odds ratio [OR] 1.10, 95% confidence interval [CI] 1.04–1.16). By grouping the time of cold ischemia by intervals, the risk of delayed graft function was greater in the 12–18 hours group (OR 2.06, 95% CI 1.02–4.15) and in the >18 hours group (OR 3.38, 95% CI 1.57–7.27). The risk of acute rejection did not increase with longer cold ischemia (p = 0.69), and cold ischemia time was not a risk factor for renal graft loss at 1-year follow-up (hazard ratio 0.97, 95% CI 0.88–1.06). In conclusion the time of cold ischemia (>12 hours) in renal transplant recipients of optimal deceased donors increases the risk of delayed graft function; however, this does not negatively impact the results in acute rejection or graft loss in the first year of the transplant.
Keywords
Acute rejection
cold ischemia time
delayed graft function
graft loss
immunosuppression
kidney transplantation
Introduction
In recent years, there has been a marked improvement in the survival of renal grafts in the first year after transplantation; however, renal graft loss, particularly in transplants from deceased donors, remains frequent.[12] Delayed graft function (DGF) is among the known risk factors for renal graft loss,[12] a complication that occurs in up to 50% of patients of deceased donors.[3456] Different risk factors have been associated with the risk of DGF, among them are the creatinine values of the donor and the cold ischemia time (CIT),[13] the latter defined as the period elapsed after the cessation of circulation, when perfusion begins with preservation solutions, until the beginning of the vascular anastomosis in the renal graft recipient.[78]
After the surgical removal of the renal graft, it is stored in a cold preservation solution to keep the renal cells viable, which does not completely prevent hypoxia-induced cell damage.[9] Additionally, the subsequent reperfusion of this ischemic kidney can induce an inflammatory and oxidative damage called “reperfusion ischemia injury,” which manifests clinically as DGF, and this in turn can decrease the survival of the renal graft.[9] Because all these events start secondary to CIT, it is reasonable to think that a prolonged CIT may further impair renal function. However, it is not completely clear if prolonged CIT should be considered as a risk factor for medium-term graft dysfunction, nor is there an agreement about what the cutoff point is in hours, after which the risk of renal graft loss increases.[10]
In the kidney transplant group of the Pablo Tobón Uribe Hospital (HPTU) a third-level complexity center in the city of Medellín, deceased donor transplants of optimal criteria predominate, where short CIT is a priority; however, some patients have prolonged CIT due to the geographical difficulty to reach quickly to the transplant. Therefore, a higher frequency of DGF is expected in this group of patients. For this reason, it is important to evaluate the relationship between CIT and DGF, taking into account other factors such as the use of new immunosuppressive therapies and the relationship of these factors with kidney graft function in the short and medium term. The failure to find a relationship between CIT and DGF and/or the presence of acute rejection (AR) or loss of the renal graft opens an opportunity to increase the number of kidney transplants that were thought should be discarded due to prolonged CIT. The objective of this study is to evaluate whether, in recipients of optimal deceased donors, the CIT is associated with an increased risk of DGF, AR, and graft loss, 1 year after kidney transplantation.
Methodology
This is a retrospective cohort in which the clinical histories of patients aged over 12 years who received a kidney transplant from a deceased donor at the HPTU were reviewed. The information was collected during the period from 2009 to 2013. Patients were excluded with a follow-up period of <1 year, those in whom insufficient information was obtained for the analysis of the data and for patients who did not receive induction therapy at the time of kidney transplantation.
Variables evaluated
The main outcome was DGF, defined as a decrease in serum creatinine of <10% per day for three consecutive days, the need for dialysis in the first week of kidney transplantation, or a serum creatinine level >3 mg/dl at the fifth day of kidney transplantation.[411] Secondary outcomes evaluated included the presence of AR confirmed by kidney biopsy and graft loss during the first year after kidney transplantation.
Type of statistical analysis
The descriptive analysis was performed according to the presence of DGF. The quantitative variables were analyzed by means and standard deviation or medians, and interquartile ranges according to the distribution of their data, which was verified using QQ graphs and histograms; the qualitative variables were analyzed as proportions. A bivariate analysis of the quantitative variables was performed using Student's t-test for independent variables or Mann-Whitney test; qualitative variables were analyzed using Chi-squared or Fisher's exact test. The relationship between prolonged CIT and DGF was evaluated by binary logistic regression; the variable CIT was introduced in the model initially as a continuous variable and subsequently as a categorical variable (0–12, >12–18, and >18 hours). The best cutoff point for CIT was sought with respect to the presence of DGF; for this, an Receiver Operating Characteristic (ROC) curve was made. Finally, the effect of CIT on renal graft dysfunction 1 year after renal transplantation was evaluated using the Kaplan–Meier survival curve and the Cox proportional hazards model. For this analysis, two definitions of the event were considered: the first definition included both patients with graft dysfunction and those who died (graft survival not censored by death); for the second definition, those patients who died with functioning kidney were considered as censored and not as events. Little difference was observed in the results using both definitions of the event; therefore, the results presented here are from the analysis using death with functioning graft as censored. A value of p < 0.05 was considered statistically significant. The statistical analyses were performed in the SPSS and STATA software.
Ethical considerations
This study was approved by the ethics and research committee of the HPTU and followed the rules on ethical aspects of research in humans contained in Resolution 008430 of 1993 of the Ministry of Health of Colombia; in addition, it preserved the confidentiality of personal data of the patients included in the study.
Results
During the 2009–2014 period, 353 patients aged between 12 and 74 years received a deceased donor kidney transplant with optimal criteria, of which 6 patients were excluded for not receiving induction therapy at the time of renal transplantation, leaving 347 patients for the analysis. The mean age at the time of transplant was 42.66 years (standard deviation [SD] ±13.32); 63.7% (n = 221) were men and in 92.4% of patients (n = 318) it was their first kidney transplant. According to the induction protocol used, 40.1% of the patients received alemtuzumab, 30.5% received thymoglobulin, and 29.1% received basiliximab. All patients received triple immunosuppressive therapy as follows: a calcineurin inhibitor (99.1%) which was cyclosporin (C2 levels 800–1200 ng/ml) in 56.2% and tacrolimus (levels 5–10 ng/ml) in 42.9%, and an antimetabolite which was mycophenolate mofetil (2 g/day) or mycophenolate sodium (1440 mg/day) in 78.7%, and azathioprine (100 mg/day) in 11%. In a small percentage of patients, instead of the antimetabolite, it was used as an mammalian target of rapamycin (mTOR), either as sirolimus or everolimus (3.47%), and all patients received steroid therapy.
The CIT in the whole population had an average of 14.4 hours (SD ±5.38), and the hot ischemia time was 34.49 minutes (SD ±8.53). The incidence of DGF and the need for dialysis during the first week after kidney transplantation was 18.4% (n = 64) and 8.1% (n = 28), respectively. Table 1 shows the baseline characteristics grouped according to the presence or absence of DGF. The univariate analysis showed that patients with DGF had a longer stay in dialysis prior to transplantation and the CIT was longer, whereas the age of the donor or the patient had no impact on the presence of DGF, nor the history of previous transplantation, transfusions, etiology of renal disease, creatinine of the donor, or the warm ischemia time.
| DGF (n=64) | AFI (n=283) | P | |
|---|---|---|---|
| Male (sex), n (%) | 42 (65.6%) | 179 (63.3%) | 0.72* |
| Mean age at transplant moment (years) (±SD) | 42.47±13.45 | 42.70±13.31 | 0.90** |
| Second transplant, n (%) | 4 (6.5%) | 22 (7.6%) | 0.75* |
| Previous transfusions, n (%) | 32 (49.2%) | 118 (41%) | 0.38* |
| Etiology of terminal kidney disease, n (%) | |||
| Unknown | 17 (26.60) | 83 (29.30) | 0.48* |
| GMN diagnosis | 12 (18.80) | 82 (29.00) | |
| Diabetes | 11 (17.20) | 35 (12.40) | |
| Polycystic kidney disease | 5 (7.80) | 20 (7.10) | |
| Urinary malformations | 7 (10.90) | 20 (7.10) | |
| Others | 12 (18.80) | 43 (15.20) | |
| Prior dialysis, n (%) | 56 (87.50) | 224 (79.20) | 0.13* |
| Donor mean age (±SD) | 33.34±13.70 | 30.83±13.45 | 0.18** |
| Mean creatinine donor (mg/dl) (±SD) | 0.89±0.28 | 0.86±0.34 | 0.50** |
| Mean cold ischemia (hours) (±SD) | 16.44±5.24 | 13.97±5.32 | 0.001** |
| Mean warm ischemia (minutes) (±SD) | 34.49±9.38 | 34.49±8.34 | 0.99** |
| Deceased donor, n (%) | 64 (100) | 278 (98.2) | 0.56* |
| Incompatibility HLA-DR, n (%) | |||
| No incompatibility | 4 (6.2) | 19 (6.7) | 0.99* |
| One incompatibility | 34 (53.1) | 149 (52.7) | |
| Two incompatibilities | 26 (40.6) | 115 (40.6) | |
| Total HLA incompatibilities, n (%) | |||
| >3 HLA incompatibilities | 55 (87.3) | 236 (83.4) | 0.44* |
| Monoclonal induction therapy, n (%) | |||
| Alemtuzumab | 19 (29.7) | 120 (42.4) | 0.06* |
| Thymoglobulin | 19 (29.7) | 88 (31.1) | |
| Basiliximab | 26 (40.6) | 75 (26.5) | |
| Immunosuppressive therapy, n (%) | |||
| MMF-TAC | 37 (57.8) | 95 (33.6) | <0.01* |
| MMF-CyA | 11 (17.2) | 127 (44.9) | |
| AZA-CyA | 5 (7.8) | 22 (7.8) | |
| MTOR-CyA | 3 (4.7) | 13 (4.6) | |
| CyA | 2 (3.1) | 12 (4.2) | |
| TAC | 2 (3.1) | 2 (0.7) | |
| MMF | 1 (1.6) | 0 | |
| MTOR-MMF | 2 (3.1) | 0 | |
| MTOR-TAC | 1 (1.6) | 1 (0.4) |
*Chi-squared test, **t-test independent samples and Fisher’s test. DGF: Delayed function of the renal graft, AFI: Adequate function of the renal graft, SD: Standard deviation, HT: Arterial hypertension, GMN: Glomerulonephritis, COAD: Chronic occlusive arterial disease, MMF: Mycophenolate, TAC: Tacrolimus, AZA: Azathioprine, CyA: Cyclosporin, MTOR: Mammalian target of rapamycin inhibitors, SD: Standard deviation, HLA-DR: Human leucocyte antigen - DR isotype
In the univariate analysis, the CIT as a continuous variable was a risk factor for DGF. When performing the multivariate analysis, despite including risk factors known as predictors of DGF (donor age, donor creatinine, number of incompatibilities, and induction therapy), the CIT remained a risk factor for DGF (odds ratio [OR] 1.10, 95% confidence interval [CI] 1.04–1.16) [Table 2]. By grouping the CIT by intervals (0–12, >12–18, and >18 hours), it was observed that the risk of DGF was increased at higher CIT (p linear-by-linear association = 0.003) [Figure 1]. Taking the 0–12 hours group as a reference value, the presence of DGF was greater in the 12–18 hours group (OR 2.06, 95% CI 1.02–4.15) and in the >18 hours group (OR 3.38, 95% CI 1.57–7.27). By analyzing the ROC curve, a CIT of 13.75 hours had a sensitivity of 73.4% and a specificity of 50% to present DGF (Area under the curve = 0.634).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Male (sex) | 1.11 | 0.63-1.96 | 0.72 | 1.09 | 0.60-2.01 | 0.78 |
| Mean age at transplant moment (years) | 0.99 | 0.98-1.02 | 0.90 | 1.001 | 0.98-1.02 | 0.99 |
| Prior dialysis | 1.84 | 0.83-4.08 | 0.13 | 1.92 | 0.83-4.42 | 0.13 |
| Cold ischemia time (hours) | 1.09 | 1.03-1.14 | 0.001 | 1.10 | 1.04-1.16 | <0.01 |
| >3 HLA incompatibilities | 1.37 | 0.61-3.06 | 0.44 | 1.52 | 0.66-3.49 | 0.33 |
| Alemtuzumab induction therapy* | 1.61 | 0.82-3.13 | 0.16 | 1.95 | 0.94-4.02 | 0.07 |
| Basiliximab induction therapy* | 0.73 | 0.37-1.47 | 0.38 | 0.80 | 0.39-1.66 | 0.55 |
*Reference induction therapy with thymoglobulin. OR: Odds ratio, CI: Confidence interval, HLA: Human leukocyte antigen
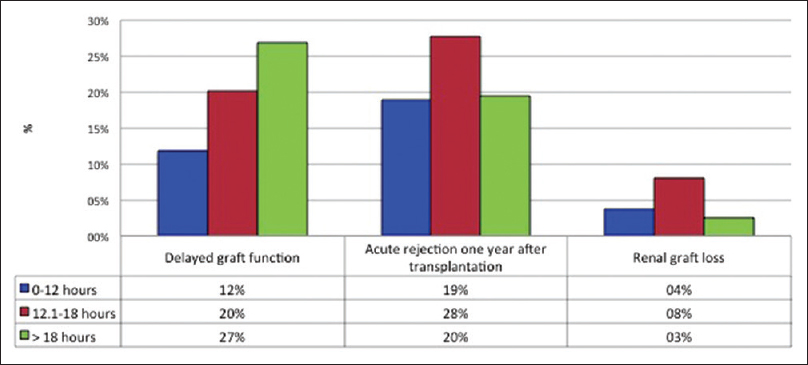
- Relationship among cold ischemia time and the presence of delayed graft function, acute rejection, and graft dysfunction 1 year after transplantation
Relationship between cold ischemia time and medium-term survival of the renal graft
Acute rejection
During the first year after renal transplantation, 22.5% of patients (n = 78) had AR, confirmed by renal biopsy, and 5.2% (n = 18) had graft loss. In the multivariate analysis, the CIT was not a risk factor for AR. Table 3 summarizes the demographic characteristics of patients with and without AR. In the univariate and multivariate analysis, older age of the recipient at the time of transplantation was associated with a decreased risk of AR; on the contrary, CIT was not a statistically significant risk factor. When stratifying the time of cold ischemia by intervals (0–12, 12–18, and >18 hours), the risk of AR did not increase with higher CIT (p linear-by-linear association: AR p = 0.69) [Figure 1].
| AR | No AR | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |||
| Male (sex), n (%) | 48 (62) | 172 (64) | 0.89 | 0.53-1.50 | 0.67 | 0.97 | 0.57-1.76 | 0.95 |
| Age at transplant moment (years), mean±SD | 39.9±13.6 | 43.4±13.2 | 0.98 | 0.96-1.00 | 0.05 | 0.98 | 0.96-0.99 | 0.05 |
| Donor age (years), mean±SD | 31.7±14.1 | 31.2±13.4 | 1.01 | 0.98-1.02 | 0.79 | NS | ||
| Second transplant, n (%) | 8 (10.5) | 18 (6.7) | 1.63 | 0.68-3.90 | 0.28 | NS | ||
| Donor creatinine (mg/dl), mean±SD | 0.9±0.33 | 0.9±0.33 | 1.36 | 0.64-2.85 | 0.42 | NS | ||
| Cold ischemia time (h), n (%) | 14.6 (4.9) | 14.4 (5.5) | 1.01 | 0.96-1.06 | 0.73 | 1.01 | 0.95-1.05 | 0.94 |
| >3 HLA incompatibilities, n (%) | 68 (88.3) | 222 (82.8) | 1.57 | 0.73-3.36 | 0.25 | 1.58 | 0.73-3.42 | 0.25 |
| Delayed graft function, n (%) | 19 (24.4) | 45 (16.8) | 1.60 | 0.87-2.93 | 0.13 | 1.44 | 0.76-2.73 | 0.27 |
| Alemtuzumab induction therapy*, n (%) | 29 (37.2) | 110 (41) | 1.15 | 0.61-2.19 | 0.66 | 1.31 | 0.67-2.57 | 0.44 |
| Basiliximab induction therapy*, n (%) | 25 (32.1) | 75 (28) | 0.91 | 0.50-1.68 | 0.77 | 0.97 | 0.52-1.81 | 0.91 |
| Thymoglobulin induction therapy, n (%) | 24 (30.8) | 83 (31) | 1 | 1 | ||||
*Reference induction therapy with thymoglobulin. AR: Acute renal rejection, OR: Odds ratio, CI: Confidence interval, SD: Standard deviation, HLA: Human leukocyte antigen, NS: Not significant
Graft loss 1 year after renal transplantation
In the univariate analysis, the survival of the renal graft (graft survival censored by death) at 6 and 12 months in patients with a CIT <14 hours was 93.6% and 92.4%, respectively. In patients with CIT ≥14 hours, graft survival at 6 and 12 months was 97.4% and 94.1%; these differences are nonstatistically significant (long-rank test = 0.80) [Figure 2]. For the Cox multivariate analysis, two models were used; in the first, only pretransplant covariables were included, adjusting for age, sex, previous dialysis history and CIT. In this model, the CIT was not a risk factor for graft loss (hazard ratio [HR] 0.98, 95% CI 0.91–1.07); in contrast, age was associated with a lower risk of graft loss (HR 0.96, 95% CI 0.93–0.99). When the supplied immunosuppressive medication and episodes of AR were included in the model, only AR was a significant risk factor (HR 20.5, 95% CI 5.97–70.45), CIT was not a risk factor for renal graft loss (HR 0.97, 95% CI 0.88–1.06).
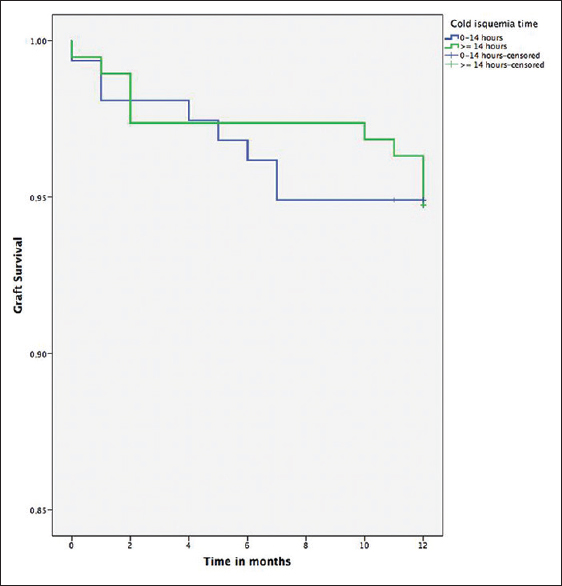
- Survival of renal graft grouped according to cold ischemia time shorter and greater than or equal to 14 hours (long rank Test = 0.80)
Table 4 describes the short- and medium-term outcomes grouped according to CIT. In Table 4, it is observed that at higher CIT, the frequency of DGF was also higher; on the contrary, no differences were observed with respect to the need for dialysis in the first week of transplantation, AR, graft loss, and serum creatinine values 1 year after renal transplantation.
| Cold ischemia time in hours | ||||
|---|---|---|---|---|
| 0-12 | >12-18 | >18 | P | |
| Delayed renal graft function, n (%) | 15 (11.3) | 28 (20.4) | 21 (27.3) | 0.01* |
| Need of dialysis during the 1st week of renal graft, n (%) | 9 (6.8) | 11 (8) | 8 (10.4) | 0.65* |
| Acute rejection 1 year later, n (%) | 25 (18.9) | 38 (27.7) | 15 (19.5) | 0.17* |
| Creatinine 1 year later (mg/ml), mean±SD | 1.39±0.89 | 1.64±1.34 | 1.45±0.98 | 0.16** |
| Loss of graft 1 year later, n (%) | 5 (3.8) | 11 (8) | 2 (2.6) | 0.15* |
| Mortality 1 year later, n (%) | 4 (3) | 2 (1.5) | 3 (3.9) | 0.52* |
*p Chi-squared test, ** Analysis of variance (ANOVA) of one factor. SD: Standard deviation
Discussion
In the evaluated cohort, a prolonged CIT was related to the risk of DGF, but not with AR or graft loss at 1 year of follow-up. Previous studies have suggested that CIT is a risk factor for DGF,[21213] which is more relevant in deceased donors whose CIT is usually longer. Ojo et al.[12] demonstrated that DGF was a risk factor for reduced renal graft survival, and CIT in turn increased the likelihood of DGF. However, its impact on medium- and long-term kidney graft survival is uncertain and this has not been extensively studied in kidney transplant patients from young donors and under current immunosuppressive therapies such as mycophenolate, tacrolimus, and induction therapy, which have achieved a reduction in the AR rate from 50% to 10–15% in the last decade.[14]
In our study, a prolonged CIT behaved as an independent risk factor for DGF, in which for each hour of CIT, the risk of DGF was increased by 10% (OR 1.10, 95% CI 1.04–1.16); however, a prolonged CIT was not a risk factor for renal graft dysfunction or AR 1 year after renal transplantation – a finding similar to that reported by previous studies.[3515] Opelz et al. reported that the increase in CIT up to 18 hours did not increase the risk of renal graft loss.[1416] Wong et al.[17] evaluated 7542 kidney transplants. They found out that a CIT >14 hours increased the risk of renal graft loss (HR 1.09, 95% CI 1.00–1.18); however, the same team did not find an association between the CIT and the function of the renal graft 12 months after the transplant, when performing the analysis only in transplant recipients of young donors (<55 years). Sert et al.[18] evaluated 111 kidney transplant patients. They found out that a prolonged CIT was associated with DGF (p = 0.018) but not with episodes of AR during the first year of kidney transplantation (p = 0.438), which is similar to what was found in our study. Xia et al. also analyzed a group of patients transplanted from deceased donors with acute kidney injury (donor creatinine >2 mg/dl). When comparing the effects of CIT, the authors found out that even with a history of acute renal injury, CIT did not increase the rejection rate during the first year after transplantation.[19]
Our findings, on the contrary, differ with other studies in which prolonged CIT had a negative effect on the long-term function of the renal graft.[202122] Morris et al.[23] evaluated 6363 deceased donor kidney transplants during the period 1986–1993. In this study, prolonged CIT increased the risk of graft loss at 1 and 5 years of follow-up. Hernández et al.[1] evaluated the impact of CIT on 829 kidneys from young donors (<50 years). Graft survival was lower in the group of patients with CIT >19 hours, compared with those who had a CIT >19 hours. Among the hypotheses that could explain the differences found in our study, we consider that universal induction accompanied by effective immunosuppressive therapies (tacrolimus and mycophenolate) in the majority of patients allow to control the immunological dysfunction secondary to a prolonged CIT,[42425] which in turn decreases the frequency of AR and renal graft loss.[12]
Additionally, a prolonged CIT combination and older donors may have a synergistic effect for a lower survival of the renal graft; in this case, kidney transplants from donors older than 55 years, with a CIT >14 hours, increased 3 times the risk of graft loss compared to recipients of younger donors, with CIT <14 hours.[16] Denecke et al.[26] suggest maintaining a CIT below 13.3 hours in patients with transplantation of donors with extended criteria; with this strategy, the authors achieved kidney graft survival times similar to those obtained in optimal donor transplantation. In our cohort, the donors were young with an average age of 33.3 years, without associated comorbidities, and without the presence of renal dysfunction during the death process (average donor creatinine before extraction: 0.86 mg/dl), which allowed a successful recovery of the renal epithelial cells after the ischemia–reperfusion injury that occurs after transplantation, without significantly affecting the function of the renal graft.[27] This could also explain the lower incidence of DGF in our cohort (18.4%), compared to previous studies.[2526] Our findings in relation to age and CIT are similar to those obtained by Lee et al.[28], who compared DGF and graft survival after 1 year among patients younger than 50 years and older than 50 years with CIT ≥24 hours; they found out better results in the kidneys of young donors, DGF of 29% vs. 42% (p < 0.01), and survival 1 year later of 84% vs. 77% (p < 0.01). Interestingly, the risk of both AR and graft loss decreased in older recipients; another study of 63,798 renal transplantation also found that the relative risk of AR was lower for recipients older than 30 years compared with recipients 18–29 years old.[29] Aging causes a decline in the immune response. Elderly patients have a smaller population of lymphocyte progenitor and decreased number of T and B cells. In addition, they have a defective responsiveness of memory T cell to CD28 costimulation; this may explain why the risk of AR decreases in older compared with younger recipients.[293031]
Our study has some limitations, such as the fact that it was performed in a single center. This is a retrospective study, which does not allow to control the variables studied and can lead to errors in the reporting of data. In addition, monitoring was performed only during 1 year. However, the data collected were reviewed by one of the researchers, therefore reducing the risk of errors in the data obtained.
Conclusion
In recipients of optimal deceased donors, prolonged CIT increases the risk of DGF; however, this was not related to short- or medium-term outcomes, such as the need for dialysis, AR, and graft loss. This is very important because, although it is clear that a prolonged CIT should not be a routine practice, kidney grafts from optimal donors with prolonged CIT (due to long distance from the recipient or donor, or depletion of the transplant group) can be considered for transplantation, independently of the CIT (not >24 hours), without negatively affecting transplant outcomes.
Financial support and sponsorship
The study was supported by HPTU, Medellin, Colombia.
Conflicts of interest
There are no conflicts of interest.
References
- Impact of cold ischemia time on renal allograft outcome using kidneys from young donors. Transpl Int. 2008;21:955-62.
- [Google Scholar]
- Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int. 2004;65:713-8.
- [Google Scholar]
- Outcomes of kidney paired donation transplants in relation to shipping and cold ischaemia time. Transpl Int. 2016;29:425-31.
- [Google Scholar]
- Neutrophil gelatinase-associated lipocalin as an early predictor of delayed graft function. Biomedica. 2016;36:213-9.
- [Google Scholar]
- Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation. 2008;85:S3-9.
- [Google Scholar]
- Biomarkers of delayed graft function as a form of acute kidney injury in kidney transplantation. Sci Rep. 2015;5:11684.
- [Google Scholar]
- Ischemia and reperfusion injury in renal transplantation: Hemodynamic and immunological paradigms. Einstein (Sao Paulo). 2015;13:129-35.
- [Google Scholar]
- Effect of cold ischaemia time on outcome after living donor renal transplantation. Br J Surg. 2016;103:1230-6.
- [Google Scholar]
- The impact of cold ischemia time on renal transplant outcome. Kidney Int. 2015;87:272-5.
- [Google Scholar]
- Factors associated with delayed graft function and their influence on outcomes of kidney transplantation. Transplant Proc. 2016;48:2267-71.
- [Google Scholar]
- Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation. 1997;63:968-74.
- [Google Scholar]
- Long cold ischemia times in same hospital deceased donor transplants. Transplantation. 2018;102:471-7.
- [Google Scholar]
- Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015;87:343-9.
- [Google Scholar]
- Influence of cold ischemia time in kidney transplants from small pediatric donors. Transplant Direct. 2017;3:e184.
- [Google Scholar]
- The impact of total ischemic time, donor age and the pathway of donor death on graft outcomes after deceased donor kidney transplantation. Transplantation. 2017;101:1152-8.
- [Google Scholar]
- The effect of cold ischemia time on delayed graft function and acute rejection in kidney transplantation. Saudi J Kidney Dis Transpl. 2014;25:960-6.
- [Google Scholar]
- Influence of cold ischemia time in combination with donor acute kidney injury on kidney transplantation outcomes. J Am Coll Surg. 2015;221:532-8.
- [Google Scholar]
- Extraction time of kidneys from deceased donors and impact on outcomes. Am J Transplant. 2016;16:700-3.
- [Google Scholar]
- Impact of cold ischemia time on initial graft function and survival rates in renal transplants from deceased donors performed in Andalusia. Transplant Proc. 2011;43:2174-6.
- [Google Scholar]
- Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:1039-47.
- [Google Scholar]
- Analysis of factors that affect outcome of primary cadaveric renal transplantation in the UK. HLA task force of the kidney advisory group of the United Kingdom transplant support service authority (UKTSSA) Lancet. 1999;354:1147-52.
- [Google Scholar]
- Induction therapies in kidney transplantation: The experience of Hospital Pablo Tobon Uribe, Medellín, Colombia 2005-2010. Transplant Proc. 2011;43:3359-63.
- [Google Scholar]
- Calcineurin inhibitor minimization in the symphony study: Observational results 3 years after transplantation. Am J Transplant. 2009;9:1876-85.
- [Google Scholar]
- Reduction of cold ischemia time and anastomosis time correlates with lower delayed graft function rates following transplantation of marginal kidneys. Ann Transplant. 2016;21:246-55.
- [Google Scholar]
- Urine neutrophil gelatinase-associated lipocalin (uNGAL) as predictor of graft dysfunction to one year of kidney transplantation. Gaceta Méd. 2018;154:275-82.
- [Google Scholar]
- The effect of age and prolonged cold ischemia times on the national allocation of cadaveric renal allografts. J Surg Res. 2000;91:83-8.
- [Google Scholar]
- Association of cold ischemia time with acute renal transplant rejection. Transplantation. 2018;102:1188-94.
- [Google Scholar]
- Immunosenescence in renal transplantation: A changing balance of innate and adaptive immunity. Curr Opin Organ Transplant. 2015;20:417-23.
- [Google Scholar]
- Aged T cells are hyporesponsive to costimulation mediated by CD28. J Immunol. 1994;152:3740-7.
- [Google Scholar]







