Translate this page into:
Tunneled central venous catheters: Experience from a single center
Address for correspondence: Dr. Krishnaswamy Sampathkumar, Department of Nephrology, Meenakshi Mission Hospital and Research Centre, Madurai - 625 107, India. E-mail: drksampath@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In the past vascular surgeons were called in to place tunneled central venous catheter (TVC) for hemodialysis patients. Advent of percutaneous technique has resulted in an increasing number of interventional nephrologists inserting it. A single centre three year audit of 100 TVCs with a cumulative follow up of 492 patient months is presented here. From 2007 to 2010, 100 TVCs were placed by nephrologists in a percutaneous fashion in the operative room or the interventional nephrology suite. Those who completed minimum of three months on the catheter were included in analysis. There were 69 males and 31 females with a mean age of 52.3±13.6 years.(range: 25-76). Chronic glomerulonephritis was the commonest cause of CKD (45%) followed by diabetes (39%).Right internal jugular vein was the preferred site (94%). TVC was utilized as the primary access to initiate dialysis in 25% of patients in whom a live donor was available for renal transplant. The blood flow was 250-270 ml/min. The Kaplan-Meier analysis showed that 3 months and 6 months catheter survival rates were 80% and 55%, respectively. The main complications were exit site blood ooze, catheter block and kink. Catheter related bacteremia rate was low at 0.4/1000 patient days. Primary cause of drop out was patient death unrelated to the TVCs. Those under the age of 40 years showed better survival, but there was no bearing of gender, catheter site, and etiology of CKD on survival. Tunneled central venous catheters could find a niche as the primary access of choice for pretransplant live donor renal transplants in view of its immediate usage, high blood flows, low infection rates and adequate patency rates for 3-6 months.
Keywords
Bacteremia
catheter survival
hemodialysis
tunneled central venous catheters
Introduction
Vascular access is the Achilles’ heel of dialysis. Creating and using an arteriovenous fistula (AVF) remains the ultimate challenge in the maintenance hemodialysis. International guidelines suggest placement of AVF in CKD stage 4. Concerned by the low AV fistula utilization the Center for Medicare and Medicaid Services in United States embarked on an ambitious initiative with a slogan “Fistula First” in 2005.[1] Despite this the use of central venous catheters has grown in USA by 55% among patients aged 22-44 and 51% and 52% among women and African Americans, respectively. The catheter use in the prevalent dialysis population has remained at 18-19% since 2003.[2] Unfortunately, ideal conditions do not exist during start of dialysis in CKD in developing countries. India has one of the largest population of advanced CKD. Creation and utilization of AVF is not without hurdles. Reliance on the limited local vascular surgical expertise results in significant primary failure rate of AVF. Most often patients present themselves in advanced uremia for the first time to the nephrologists without a functioning AVF. Extremely high rates of bacteremia are encountered within 2-4 weeks in nontunnelled. To circumvent these problems for the last 3 years we have started placing tunneled venous catheters (TVC) in our unit's interventional nephrology suite. This communication describes the short and long term success rates of such catheters.
Patients and Methods
The following patients with CKD were selected for TVC placement: those who had exhausted other accesses including the AVF, those waiting for maturation of AVF and as primary access for hemodialysis if they had a living related renal donor. Evaluation of the internal jugular vein was done by ultrasound to demonstrate the relation of the vein with the adjoining artery, its size, patency, and absence of intraluminal clots. The site was cleaned and draped in a sterile fashion. Conscious sedation was administered during the procedure with 1-2 mg of midazolam and 50-100 mcg of fentanyl. The patients were monitored closely during the entire procedure for pain and cardiac arrhythmias. The vein was accessed using anatomical landmarks or real time ultrasound guidance using a thin 18 gauge needle followed by 16 gauge needle through which a guide wire was advanced. Next the catheter was tunneled using the tunneling device from the manufacturer's kit with exit site 4-5 cm below the clavicle in the infra clavicular fossa. After sequential dilatation of the venotomy site, a peel away sheath was introduced. The guide wire was removed and the proximal end of the tunneled catheter was then advanced through the sheath to the superior vena cava. Care was taken to peel the sheath outside the body, to avoid any venous tear. The tip of the catheter was confirmed to be positioned at right atrium or the SVC/right atrial junction with fluoroscopy. Three T's were checked viz., position of its tip, its top (curvature in the neck) and tug (free aspiration of blood through a 10 cc syringe). The catheter was locked with appropriate volume of heparinized saline. The catheter was anchored with sutures at the exit site and the butterfly wings. The skin incision over venotomy site was sutured with two interrupted stitches and pressure dressing applied for 24 h. The patient was ready for hemodialysis immediately. After each dialysis session an antibiotic lock solution containing 10 mg/ml of vancomycin, 5mg/ml of gentamicin, and 1000 units/ ml of heparin was injected in the arterial and venous limbs of the catheter. Dialysis staff followed universal infection control practices while caring for the catheters. Disposable sterile gloves were used for the connection and disconnection. The connection ports were covered with gauze soaked in betadine solution during dialysis.
In the initial 24 patients we used permcath TVCs. The blood loss during the insertion of this catheter was significant and undesirable given the high incidence of anemia in these patients. We later switched to inserting 14.5 F Mahurkar Maxid (Tyco Healthcare/Covidien) cuffed dual lumen TVCs with end holes and a guard over the peel away sheath which prevents undue blood loss. Catheter flow rates, patency, catheter survival, and catheter related infections were collected from the hemodialysis run sheets and patients’ medical records. Patient demographics, technical complications, and reasons for catheter removal were recorded.
Results
One hundred consecutive patients in whom the TVCs were placed form the study population. The demographic profile, site and indications are provided in the Table 1. Figure 1 depicts the age distribution of the cohort.

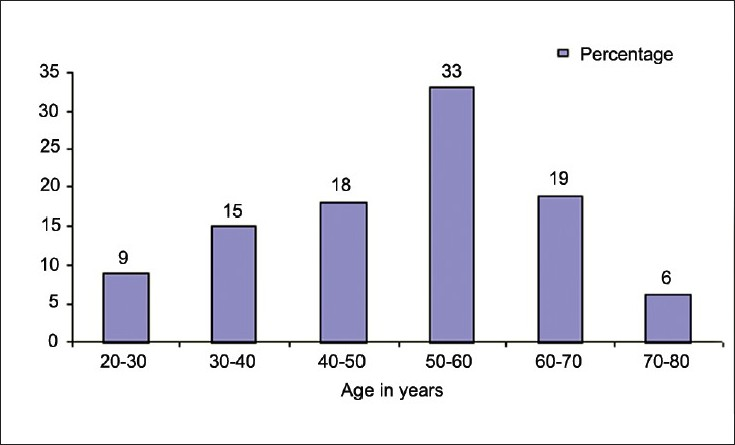
- Age group of patients
As seen from Figure 1, about a third of the patients belong to the age group of 50-60 years. In this age group, the indication was either a failed AVF or as a bridge during AVF maturation. In another much younger group it was utilized as the primary prerenal transplant access [Table 2].

In the initial period of our study the predominant indication was for a failed AVF (49%). In 26 patients, it was used as a bridge access before use of AVF. As our experience grew we realized that it can be utilized as the primary access for pre transplant HD in whom a live-related donor is available. Hence, in 25 patients it was utilized in that fashion. The range of blood flows utilized was in the range of 250-300 ml/min with a mean flow of 275 ml/min.
The most common complication was the exit site ooze of blood which could be controlled with compression dressing. There were six bacteremic episodes in three patients (of two each). The blood cultures were negative in two instances. It grew Staphylococcus aureus (2), Klebsiella (1), and Escherichia coli (1) in the other four. All episodes occurred during or immediately after the dialysis procedure. These were treated with antibiotics according to sensitivity patterns for 2 weeks. TVCs were removed after the second episode in 48 h in all three instances. Two instances of catheter malfunction due to kinking were set right by open surgical mode with the help of vascular surgeons. Both occurred in the initial five cases after which no surgical intervention or consult was required. In three instances of catheter block due to intraluminal clot, urokinase infusion was temporarily successful inrecanalization. However, ultimately two of the catheters had to be removed [Table 3].

At the time of writing, 55 of these catheters were functioning and 45 were removed with a median period of 7 months. Drop outs occurred predominantly due to death with functioning TVCs due to associated comorbidities in 23 cases [Table 4]. Eleven patients underwent renal transplantation. In three instances the catheter sepsis was the cause for its removal. All three survived. In four cases the patients were taken up for dialysis through AVF and two cases were switched to continuous ambulatory peritoneal dialysis. Two permanently blocked catheters were removed.

Analysis of the factors affecting the longevity of catheters showed that there was no influence of sex, underlying disease and site of placement. Age below 40 years had a significant impact on the survival by Chi-square test (P = 0.023).
Unadjusted Kaplan–Meier survival analysis showed 80% and 55% catheter survival rates in 3 and 6 months, respectively [Figure 2, Table 5].

- Kaplan-Meier survival graph of TVCs
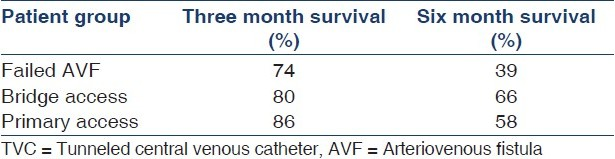
Sub group analysis of catheter survival in three groups of patients are given in Table 5.
Discussion
Tunneled catheters play an important role by providing a viable dialysis access for maintenance hemodialysis. Dialysis Outcomes and Practice Patterns Study (DOPPS) analysis showed that catheters as a primary vascular access were used in 23%-73% of incident dialysis patients in several countries.[1] The trend has not changed over the last decade. The 2005 United States Renal Data Systems (USRDS) data show that catheter utilization remains at a high level. The situation in India is far from satisfactory[2] Only 15% of patients with stage 5 CKD who were admitted to a large referral nephrology hospital in Vellore, India had a functioning AVF before start of hemodialysis.[3] Eighty five percent of these patients had to be started on HD before a fistula could mature through a central venous non tunneled catheters. The incidence of bacteremia is extremely high in these catheters given the sub optimal living conditions of these patients coupled with the hot and humid climate predisposing towards catheter colonization from skin pathogens. The advantages of TVCs include short learning period for the nephrologist who is already well versed with placement of central venous catheters, availability of multiple sites for catheter insertion, immediate availability of an access providing high blood flow rates of 250-300 ml/minute, no hemodynamic consequences unlike AVF, its longevity and the low rates of catheter infection. The interventional nephrology suite positioned close to the hemodialysis room provides ideal infrastructure for this procedure. Cannulation is best performed under ultrasound guidance. Anatomical variations of the jugular veins are present in 5–18% of patients and ultrasound guided placement results in significantly lesser incidence of failed attempts and arterial puncture.[4–6] In our case series all catheters were inserted by only the nephrologist either in the interventional nephrology suite or in the fluoroscopy unit. Fluoroscopy guidance was required only in eight cases (8%). In comparison a unit from UK had three types of insertion. In 358 cases, the TVCs were inserted in an operating theatre setting by a surgeon almost exclusively using fluoroscopic guidance. In other 454 cases a dedicated procedures room on the acute nephrology ward, with monitoring (blood pressure, pulse oximetry, and electrocardiography) but no fluoroscopic guidance. In the remaining cases the catheters were inserted under fluoroscopic guidance by a physician in the radiology angiographic suite.[7]
The published complications of hemothorax, pneumothorax, venous rupture, and atrial perforation were not seen in our series. In eight cases oozing at the tunnel site entry was encountered which resolved on conservative management. In studies looking at the luminal colonization rates for the central venous catheters it is recognized that by 16 weeks almost all the catheters are colonized.[8] In our series we have observed a low bacteremia rate, comparable to other studies.[9–12] This is attributable to the routine use of antibiotic lock solution made of vancomycin and gentamicin as suggested elsewhere.[1013] A metaanalysis evaluated the incidence of catheter associated bacteremia in 29 trials with 2886 patients and 3005 catheters. Antimicrobial catheter locks significantly reduced the rates (rate ratio, 0.33, 95% CI 0.24-0.45) and exit-site infections (rate ratio 0.67, 95% CI 0.47-0.96).[11] Partial catheter block due to thrombosis was treated with urokinase therapy as described elsewhere.[14] Death with a functioning catheter was the commonest cause of drop out which was followed by renal transplant and switch to AVF. Sepsis and malfunction of catheters accounted for catheter removal in three and two cases, respectively.
Studies looking in to factors affecting the catheter survival are emerging. A multicentric analysis from United Kingdom showed a significant catheter survival advantage of first catheters over second and subsequent insertions, of right internal jugular site over left internal jugular and non-diabetic over diabetic patients. Patient age, sex and operator (physician in ward-based procedure room under ultrasound control or surgeon in operating theatre under fluoroscopic assistance) did not significantly affect survival. The Permcath design demonstrated inferior survival in all but first catheter insertions in catheter-naive patients. The HemoSplit and Tesio twin catheter designs demonstrated best survival overall. By Cox proportional hazard modeling the design and the position of the TVC seemed to be the most significant independent survival factors.[7] A centre from Italy has reported their 10-year experience of TVCs. Over 450 central venous catheters were inserted with a satisfactory survival rate of 86% at 1 year and 79% at 2 years[12] There has been a renewed interest in their usefulness in maintenance hemodialysis setting as evidenced by a number of publications on this procedure.[15–18] In 463 incident patients admitted to a premier tertiary care unit in India, the median duration to live donor transplant was 93 days.[19]
We suggest that TVCs could be utilized as a primary access in patients presenting to a renal transplant unit with a potential living related donor. This is based upon our results showing 100% utilization rates of TVCs with high blood flow rates and negligible rates of bacteremia in the first 6 months. In contrast the failure rates of AVF ranges between 15% and 40% and the maturation time is typically between 6 and 8 weeks.
On the flip side, mention has to be made of CHOICE study which showed that starting HD programs through a central catheter, when compared with starting HD through an AVF, increased mortality risk by 30%-50% after adjusting for various variables.[20] One could argue that this increased mortality risk associated with catheters does not depend solely on the catheter itself but rather on the fact that patients who have a catheter also have a precarious cardiovascular status, related to older age and greater comorbidity that first prevents the construction of the AVF and, secondly, confers increased mortality.
Conclusion
Our three years experience of TVCs in which 100 catheters were inserted and utilized for maintenance hemodialysis has shown catheter survival rates of 80% at three months and 55% at six months. The catheter related bacteremia rates were low. We suggest a niche for them as the primary pretransplant access in those who present in advanced stages of CKD with a potential living donor since hemodialysis can be carried over up to renal transplantation without recourse to AV fistula.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48:S248-73.
- [Google Scholar]
- 2009 Annual Data Report: Atlas of chronic kidney disease and end – stage renal disease in the United States. Am J Kidney Dis. 2010;55:S266-7.
- [Google Scholar]
- Pre-tertiary hospital care of patients with chronic kidney disease in India. Indian J Med Res. 2007;126:28-33.
- [Google Scholar]
- Anatomical variations of internal jugular vein location: Impact on central venous access. Crit Care Med. 1991;19:1516-9.
- [Google Scholar]
- Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: An ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13:134-8.
- [Google Scholar]
- Ultrasound-assisted cannulation of the internal jugular vein.A prospective comparison to the external landmark-guided technique. Circulation. 1993;87:1557-62.
- [Google Scholar]
- Factors affecting long-term survival of tunnelled haemodialysis catheters-a prospective audit of 812 tunnelled catheters. Nephrol Dial Transplant. 2008;23:275-81.
- [Google Scholar]
- A prospective study of central venous hemodialysis catheter colonization and peripheral bacteremia. Clin Nephrol. 1999;19:265-79.
- [Google Scholar]
- Catheter management protocol for catheterrelated bacteremia prophylaxis. Semin Dial. 2003;16:403-5.
- [Google Scholar]
- Prevention of tunnelled hemodialysis catheter-related infections using catheterrestricted filling with gentamicin and citrate: A randomized controlled study. J Am Soc Nephrol. 2002;13:2133-9.
- [Google Scholar]
- Systematic review of antimicrobials for the prevention of haemodialysis catheter-related infections. Nephrol Dial Transplant. 2009;24:3763-74.
- [Google Scholar]
- Do central venous catheters have advantages over arteriovenous fistulas or grafts? J Nephrol. 2006;19:265-79.
- [Google Scholar]
- Tunneled catheter-antibiotic lock therapy for prevention of dialysis catheter-related infections: A single center experience. Saudi J Kidney Dis Transpl. 2008;19:593-602.
- [Google Scholar]
- Urokinase for restoring patency of malfunctioning or blocked central venous catheters in children with hemato-oncological diseases. Support Care Cancer. 2004;12:840-3.
- [Google Scholar]
- Implantation of permanent jugular catheters in patients on regular dialysis treatment: Ten years’ experience. J Vasc Access. 2001;2:68-72.
- [Google Scholar]
- Permanent double-lumen central venous catheters--replacement for arteriovenous fistula. Med Arh. 2006;60:22-5.
- [Google Scholar]
- A prospective comparison of two types of tunneled hemodialysis catheters: The Ash Split versus the PermCath. Cardiovasc Intervent Radiol. 2005;28:23-9.
- [Google Scholar]
- Haemodialysis for end-stage renal disease in Southern India--a perspective from a tertiary referral care centre. Nephrol Dial Transplant. 1998;13:2494-500.
- [Google Scholar]
- for the CHOICE study. Type of vascular access and survival among incident hemodialysis patients: The Choices for Healthy Outcomes In Caring ESRD (CHOICE) study. J Am Soc Nephrol. 2005;16:1449-55.
- [Google Scholar]







