Translate this page into:
Ultrasound-Guided Percutaneous Peritoneal Dialysis Catheter Insertion for Urgent-Start Dialysis: Technique Description and Experience of a Single Center in Mexico City
Corresponding author: Froylan David Martínez-Sánchez, Department of Internal Medicine, Hospital General Dr. Manuel Gea Gonzalez, Calz. de Tlalpan 4800, Belisario Domínguez Secc 16, Tlalpan, 14080 Ciudad de México, Mexico. E-mail: froylan.martinez@comunidad.unam.mx
-
Received: ,
Accepted: ,
How to cite this article: Balderas-Juarez J, Salinas-Ramirez MA, Hernandez-Castillo JL, Moreno-Novales R, Cortina-Marquez RA, Martinez-Sanchez FD. Ultrasound-Guided Percutaneous Peritoneal Dialysis Catheter Insertion for Urgent-Start Dialysis: Technique Description and Experience of a Single Center in Mexico City. Indian J Nephrol. doi: 10.25259/IJN_256_2024
Abstract
Background
Urgent-start peritoneal dialysis (PD) is a practical and useful form of renal replacement therapy (RRT). The main methods used for PD catheter placement include open/laparoscopic surgery as well as percutaneous puncture using the Seldinger technique. Placing PD catheters using ultrasound-guided percutaneous techniques could reduce some complications associated with the blind technique. Hence, using the Argyle Dialysis Catheter Kit, we adapted a technique for ultrasound-guided percutaneous placement of PD catheters. This study aims to describe our technique and outcomes in urgent-start PD.
Materials and Methods
Data were collected retrospectively on all patients who underwent PD catheter insertion by a nephrologist in urgent-start PD. All catheters were evaluated for complications from insertion until discharge from the hospital.
Results
This technique was performed in 74 patients with urgent-start RRT. The mean age of the subjects was 54.2 ± 14.6 years, and 40.5% were women. The mean body mass index (BMI) was 26.4 ± 5 kg/m2, and 23% of the patients had a BMI of > 30 kg/m2. A history of abdominal surgery was noted in 23%. No patient experienced tunnel infection or bowel perforation. Patients experienced transient PD dysfunction (21.6%), catheter migration (20.3%), leakage (8.1%), and peritonitis (13.5%). All complications were resolved before discharge.
Conclusion
Our adapted ultrasound-guided PD catheter placement technique with a prespecified kit was demonstrated to be safe and useful in patients with increased adiposity and/or a history of previous abdominal surgery. This procedure could reduce in-hospital costs for patients with end-stage kidney disease.
Keywords
Urgent-start peritoneal dialysis
Peritoneal dialysis catheter
Percutaneous technique
Ultrasound
Introduction
Peritoneal dialysis (PD) is a form of renal replacement therapy (RRT) that can be used for urgent-start dialysis, offering numerous advantages.1 It allows patients to undergo home therapy, enhancing their quality of life and independence.2 PD provides continuous treatment, reducing the risk of vascular complications and minimizing interruptions in therapy.3 PD has been shown to increase survival rates, improve quality of life, and enhance satisfaction rates at lower costs than hemodialysis (HD).2 Consequently, PD is regarded as a safe and cost-effective RTT, particularly beneficial in resource-limited countries such as Mexico.4
The predominant methods used for PD catheter placement include open or laparoscopic surgery as well as percutaneous puncture using the Seldinger technique.5,6 Percutaneous catheter placement is a bedside operative technique primarily performed by nephrologists.6 This method is characterized by its simplicity and confirmed efficacy.3 Percutaneous placement of PD catheters is conducted under local anesthesia with minimal transcutaneous access, facilitating rapid recovery.3,7 A limitation of the blind Seldinger technique is its inability to provide direct visualization of the pelvic cavity.8 Consequently, accurately placing the PD catheter in the appropriate position based solely on tactile feedback can be challenging.8,9 As a result, there is a risk of the catheter being inserted too deeply, which increases the likelihood of catheter-associated complications such as bowel perforation, leakage, and abdominal discomfort.8,10
Placement of PD catheters using ultrasound-guided percutaneous techniques offers real-time imaging of the procedure,11 allowing for direct observation of the catheter and potentially reducing complications associated with the blind Seldinger technique.8 In real-world clinical settings, patients who have undergone previous surgery or are obese are typically directed toward laparoscopic placement.9,12 Conversely, individuals of advanced age or those with multiple comorbidities are often guided toward percutaneous image-guided placement.9 This preference is due to the presence of factors that disqualify these patients from receiving general anesthesia.12
At Hospital General Dr. Manuel Gea González, the authors have adapted a technique for ultrasound-guided percutaneous placement of PD catheters using the Argyle Dialysis Catheter Kit. The objective of this study is to describe the technique and to report our single-center experience.
Materials and Methods
This was a single-center cross-sectional observational study that included 74 patients with end-stage kidney disease who required urgent-start RRT from January 2022 to December 2023 at the Department of Internal Medicine, Hospital General Dr. Manuel Gea González, Mexico City, Mexico. The inclusion criteria were individuals aged between 18 and 90 years of all genders diagnosed with chronic kidney disease (CKD), presenting with CKD stage 5 without a plan for dialysis modality, and consenting to peritoneal catheter placement. This study focused exclusively on patients requiring urgent-start PD, where dialysis was initiated immediately or within 48 hours following catheter insertion. This approach was necessitated by the patient’s immediate need for RTT, so a lead-in period of one to two weeks was not utilized. This study was approved by the HGDMGG Research Committee and Research Ethics Committee (REF 14-69-2024), and patient anonymity was guaranteed following the 1975 Declaration of Helsinki. Upon admission, the patient or a family member signed an informed consent form permitting the use of their medical information for didactic, research, and publication purposes.
Urgent-start PD was defined as initiating PD within 48 hours of catheter insertion in patients presenting with end-stage kidney disease requiring immediate RRT. Catheter dysfunction was defined as any complication that resulted in prolonged in and out dialysis solution times during PD. Catheter migration was defined as the displacement of the PD catheter from its intended position in the pelvic cavity. To resolve this issue, we administered prokinetic drugs or encouraged patients to walk or move out of bed. This was identified using imaging techniques, such as X-ray, when the catheter was found outside the pelvic cavity. Pericatheter leakage was defined as the escape of dialysis solution around the PD catheter insertion site.
The equipment used for all the procedures was an Argyle (Medtronic, USA) Peritoneal Dialysis Catheter Kit, a coil 2-cuff Tenckhoff peritoneal dialysis catheter, and a Siemens ACUSON Juniper Ultrasound System (Siemens Healthineers, Germany). In this study, only coiled Tenckhoff peritoneal dialysis catheters were used.
The usual preoperative protocol involved patients undergoing routine tests, including complete blood count, coagulation, liver and kidney function, and serum electrolyte analysis. Subsequently, the medical staff explained the procedure’s benefits and risks to the patient and their family. Following this discussion, the patient or a family member provided informed consent for the procedure. For the upcoming procedure, oral anticoagulants and antiplatelet agents were discontinued five days before the scheduled date. Furthermore, heparin was stopped 12 hours before the procedure. As part of the intestinal preparation protocol, patients are advised to take sennosides (8.6 mg tablet) orally twice daily and mosapride (2.5–5 mg tablet) orally every eight hours, starting 24–48 hours before the procedure.
Additionally, polyethylene glycol should be ingested as one sachet dissolved in 250ml of liquid, taken four times 12 hours before the procedure, to prevent abdominal distension. In the event of incomplete bowel emptying, a 250 ml evacuating enema with injectable water at 35°C should be administered. A prophylactic antimicrobial should be administered intravenously one hour before the procedure, with options including Cephalothin 1 g, Cefotaxime 1 g, Cefuroxime 1 g, or Ceftriaxone 1 g.
Technique description
Abdominal pre-marking
The pre-marking process begins by locating the pubic symphysis. A Tenckhoff catheter is used as a guide to align the upper edge of the PD catheter with the upper edge of the pubic symphysis [Figure 1]. We then proceed to mark the two cuffs. The first cuff determines the incision site, typically located 2–4 cm lateral to the midline. The second cuff serves as a guide for marking the tunnel. Using a sterile marker, we draw the catheter curve positioned 1–2 cm above the second cuff, creating a 90° angle. We mark the exit site of the PD catheter at an approximate angle of 30°. The superficial cuff (second cuff) is marked on the curve, ensuring a 2–4 cm distance between the superficial cuff and the exit site [Figure 2].
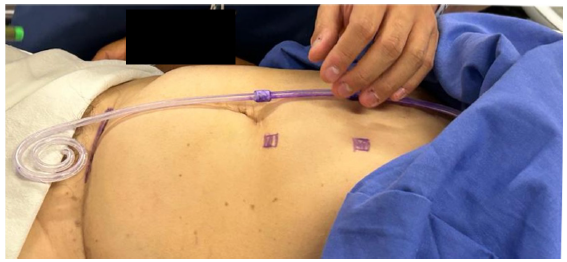
- Location of the pubic symphysis, utilizing a Tenckhoff catheter as a guide for marking.
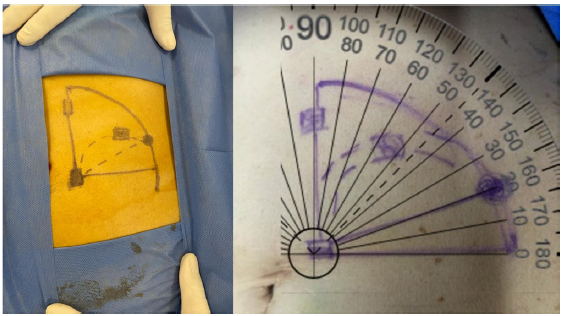
- Catheter curve marking.
Pre-procedure ultrasonographic tracking
The pre-procedure ultrasonographic tracking aims to locate the anatomical structures through which the needle will pass. Measuring the depth of the abdominal wall to the peritoneum and identifying the epigastric vessels is crucial to avoid puncture or injury. Ultrasound guidance helps direct the needle into the abdominal cavity, preventing injury to the epigastric vessels and perforation of intestinal loops. Once the peritoneal cavity is reached, real-time ultrasound tracking helps visualize fluid infusion into the peritoneal cavity. A high-frequency linear ultrasound (5 MHz) is used to identify the skin, adipose tissue, fascia and rectus muscle, peritoneum, intestinal wall, and epigastric vessels [Figure 3].
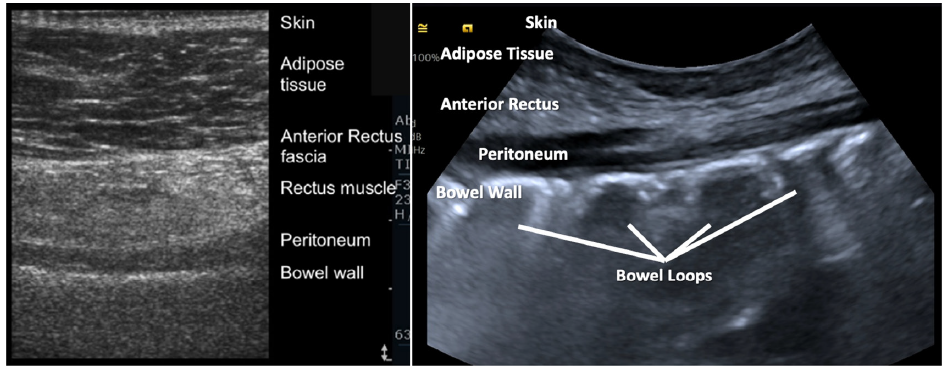
- Pre-procedure ultrasonographic tracking.
Percutaneous procedure
The procedure starts with asepsis and antisepsis, followed by the injection of 10 ml of 2% Lidocaine at the site marked for the first cuff. After the Lidocaine injection, a 1–2 cm incision is made in the skin and subcutaneous tissue at the first cuff marking site. Dissection with straight Kelly forceps is performed until reaching the fascia of the rectus abdominis muscle, being careful not to dissect or perforate the muscle.
The needle from the percutaneous Tenckhoff catheter placement kit is mounted onto an intravenous infusion set containing either 1000 mL of 0.9% saline solution or Hartmann’s solution. Under ultrasound guidance, the needle is used to puncture the rectus abdominis muscle initially at a 90° angle and then redirected to a 45° angle. Saline solution infusion begins once the needle tip is visualized in the peritoneum [Figure 4]. Flow is verified using Color Doppler ultrasound and observed as a steady stream in the drip chamber of the standard drip set.
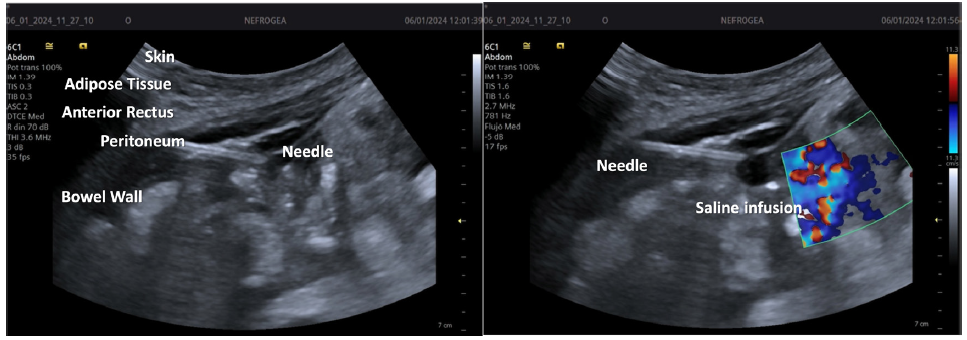
- Visualization and verification of the catheter needle.
Next, the metal guidewire is advanced into the peritoneal cavity, followed by the insertion of the dilator. The dilator is removed and the outer sheath is left in place. The catheter, with the stylet, is inserted through the outer sheath to the first cuff, and the stylet is then removed. The outer sheath is fractured, the first cuff is inserted into the peritoneal cavity through the rectus abdominis muscle using straight forceps, and the outer sheath is removed.
After connecting the transfer set to the Tenckhoff catheter for the initial exchange of dialysis solution (1.5% 2000 mL) from inflow to outflow, the entry of dialysis fluid into the peritoneal cavity is verified using ultrasound. The time for both inflow and outflow is recorded. The tunneling technique is performed after removing the connector with the transfer set. Lidocaine is infiltrated at the tunneling site and the catheter exit site. The tunneler is placed on the Tenckhoff catheter and subcutaneous tunneling is performed with the exit at the marked point. Finally, the dialysis fluid flow is verified and the titanium connector and transfer set are placed.
Statistical analysis
Statistical analysis was performed using SPSS 26 (SPSS Inc., Chicago, IL). Data were screened for outliers and normality assumptions. The normality of continuous variables was assessed with the Shapiro-Wilk normality test and visually using histograms and Q-Q plots. Values are expressed as mean ± standard deviation, median (interquartile range), or frequencies (%).
Results
The technique was performed in 74 patients with end-stage kidney disease with urgent-start RRT. The mean age of the subjects was 54.2 ± 14.6 years, and 40.5% (n = 30) were women. The mean body mass index (BMI) was 26.4 ± 5 kg/m2, and 23% of the patients had a BMI >30 kg/m2. Likewise, 71.6% had type 2 diabetes, 82.2% had hypertension, and 23% had a history of abdominal surgery.
All catheters were placed during hospitalization. The median in-hospital stay was 15 (12–18) days. Only one patient died during hospitalization. The mean duration of the procedure from skin sterilization to the end of insertion was 54 ± 14 minutes. No patient experienced tunnel infection or bowel perforation. Transient PD dysfunction was present in 21.6%, catheter migration in 20.3%, pericatheter leakage in 8.1%, and PD-related peritonitis in 13.5%. The time between the catheter placement and the first PD session was < 12 hours in 66.2%, 12–24 hours in 24.3%, 24–48 hours in 5.4%, and more than 48 hours in 6.8%. It is worth mentioning that upon discharge, all PD catheters with dysfunction were resolved. Despite ultrasound guidance, which facilitated accurate initial placement of the catheter tip within the true pelvis, migration still occurred in some cases.
We conducted a subgroup analysis to evaluate the complication rates specifically among patients with a BMI > 30 kg/m2 and those with a history of abdominal surgeries.
Patients with obesity (BMI > 30 kg/m2): Among the 17 patients with a BMI of > 30 kg/m2, the complication rates were as follows: transient PD dysfunction occurred in 23.5% of cases, catheter migration in 17.6%, pericatheter leakage in 11.8%, and peritonitis in 17.6%. These rates are comparable to the overall study population, suggesting that obesity did not significantly increase the risk of complications with the ultrasound-guided technique.
Patients with a history of abdominal surgeries: Among the 17 patients with a history of abdominal surgeries, the complication rates were: transient PD dysfunction in 29.4%, catheter migration in 23.5%, pericatheter leakage in 5.9%, and peritonitis in 11.8%. These rates suggest that while a history of abdominal surgery may slightly increase the risk of transient PD dysfunction and catheter migration, the use of ultrasound guidance mitigated these risks, allowing for safe catheter placement.
In addition to the immediate post-procedure outcomes, we followed up with all 74 patients for six months. During this time, no patient experienced catheter dysfunction (any complication resulting in prolonged in and out dialysis solution times during PD). Nonetheless, peritonitis was observed in 16.44% (n = 12) of patients, while 83.56% (n = 61) did not develop peritonitis. Catheter removal was necessary in 6.85% (n = 5) of patients, with the remaining 93.15% (n = 68) retaining their catheters. These results suggest that the ultrasound-guided percutaneous catheter placement technique provided immediate efficacy and maintained safety and functionality over the short-term period.
Discussion
The contemporary use of PD is supported by improvements in overall mortality rates, better survival rates during the initial years on dialysis, and comparable long-term survival rates to HD.13 Patients with CKD and end-stage kidney disease requiring urgent RRT may opt for PD, HD, or preemptive renal transplantation.14 PD is significantly cheaper than HD.10 PD catheters can be placed either in the operating room by surgeons or at the bedside by nephrologists, which offers practical and cost-saving advantages.8,12 In this study, we report on our adapted technique, refined over the last three years. Using ultrasound-guided imaging can prevent complications associated with the blind Seldinger technique.6 We aimed to report our technique and experience, analyzing its advantages compared to other techniques or laparoscopic surgery. All patients in the study underwent urgent-start PD, allowing us to assess its efficacy and safety. The six-month follow-up data strengthens the findings of this study, demonstrating that the ultrasound-guided percutaneous PD catheter placement technique is effective and safe in both the immediate and short-term periods. The absence of catheter dysfunction, the relatively low incidence of peritonitis, and the high retention rate of the catheters at six months further validate the utility of this technique in urgent-start PD. These results support the technique’s use in clinical practice, particularly for patients requiring urgent-start dialysis. Future studies with extended follow-up periods could provide further insights into this approach’s long-term outcomes and durability.
Urgent-start PD has been associated with a better quality of life, a lower incidence of catheter-related infections, and no difference in mortality compared to HD.1,14 Data from Huang et al. showed that ultrasound guidance provided a 100% catheterization success rate and lower frequencies of catheter-related complications.8 However, our study had a larger number of patients (74 vs. 34), a higher BMI (26.4 ± 5 vs. 20.8 ± 3.6 kg/m2), and a younger population. We did not observe any complications during the procedure compared to a 17% rectus muscle hemorrhage incidence rate in the other study. Overall, both studies demonstrated the safety and practicality of ultrasound-guided percutaneous PD catheter placement. Using ultrasound guidance in our technique provided real-time visualization, which was instrumental in ensuring the catheter tip reached the true pelvis. This likely contributed to reducing the risk of catheter dysfunction due to improper placement. However, catheter migration was observed in 20.3% of cases, indicating that ultrasound improves placement accuracy, but it does not entirely prevent migration. Further studies could explore additional measures to reduce migration rates in urgent-start PD patients.
Methods for placing PD catheters include traditional open surgery, laparoscopic surgery, and the Seldinger technique.6,12 Open surgery, while common, has high postoperative complications, with catheter displacement occurring in 10–20% of cases.15 Laparoscopic placement requires general anesthesia and advanced equipment, leading to higher costs.5,8 Ma et al. found that patients with a history of abdominal surgery often underwent open surgery. Still, in our population, patients underwent the percutaneous technique based on clinical criteria and pre-procedure ultrasonographic evaluation.6 Recent studies suggest that a history of abdominal surgery is no longer a contraindication for the ultrasound-guided percutaneous technique.1,9,10 Using ultrasound guidance in our technique provides significant safety advantages, particularly in preventing bowel perforation. While traditional methods, such as applying positive pressure through a suction needle, can help confirm the needle’s placement in the peritoneal cavity, ultrasound offers real-time visualization of the needle’s trajectory, allowing for precise navigation around bowel loops and blood vessels. This visual guidance reduces the risk of misplacement and minimizes the potential for serious complications such as bowel perforation and vascular injury.
Infectious complications are common in PD.9 A meta-analysis by Esagian et al. reported a 23.6% rate of infectious complications with percutaneous PD catheter placement compared to 28.5% with surgical placement.12 The data quality is generally poor, but lower rates of tunnel and exit-site infections were noted with the percutaneous technique.12 Surgical placement may predispose patients to infections due to a more significant entry point for microorganisms.1,9
This work has some strengths to report. It describes an adapted percutaneous technique using ultrasound and a prespecified kit for patients with urgent-start PD. We acknowledge that common PD access studies should provide follow-up beyond the immediate perioperative period to assess the procedure’s effectiveness. We are completing the long-term follow-up of these patients to report on its effectiveness. Longer follow-ups also allow for the comparison of outcomes to recognized clinical goals.
Moreover, our population had a mean BMI higher than 25 kg/m2, with one-fifth living with obesity. Limitations include being a single-center retrospective study in Mexico, which may affect generalizability and the lack of comparison with open or laparoscopic surgery. Future studies are needed to confirm our findings.
Conclusion
We have reported an adapted ultrasound-guided PD catheter placement technique with a prespecified kit in urgent-start PD. This procedure is safe and practical in patients with increased adiposity and a history of previous abdominal surgery. It could reduce in-hospital costs for patients with end-stage kidney disease. Our subgroup analysis demonstrated that the ultrasound-guided percutaneous technique was effective even in higher-risk populations, such as those with obesity or a history of abdominal surgeries. While there were some variations in complication rates, these differences were not statistically significant, underscoring the safety and utility of this technique in diverse patient groups. The short-term follow-up data, extending to six months post-procedure, indicates that this technique is immediately effective and reliable over time with minimal complications.
Availability of data and materials
All data and materials are available from the corresponding author and will be made available at a reasonable request.
Acknowledgments
The authors would like to thank the head of the Department of Internal Medicine for acquiring the Siemens ACUSON Juniper Ultrasound System. Previously, the authors placed catheters using the blind Seldinger technique. Partial data were presented at the 2023 World Congress of Nephrology as WCN23-0645: Early Peritoneal Dialysis Therapy After Catheter Placement in Decompensated End-Stage Chronic Kidney Disease: Experience of a Single Referral Center in Mexico.
Conflicts of interest
There are no conflicts of interest.
References
- Factors associated with urgent-start peritoneal dialysis catheter complications in ESRD. Kidney Int Rep. 2020;5:1722-28.
- [PubMed] [Google Scholar]
- Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: A systematic review and meta-analysis. Kidney Blood Press Res. 2017;42:717-27.
- [CrossRef] [PubMed] [Google Scholar]
- Best practices consensus protocol for peritoneal dialysis catheter placement by interventional radiologists. Perit Dial Int. 2014;34:481-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Challenges for peritoneal dialysis centers before and during the COVID-19 pandemic in Mexico. Arch Med Res. 2022;53:431-40.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of fluoroscopic and ultrasound-guided placement versus laparoscopic placement of peritoneal dialysis catheters. Clin Kidney J. 2018;11:549-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association between different peritoneal dialysis catheter placement methods and short-term postoperative complications. BMC Nephrol. 2021;22:151.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Image-guided approach to peritoneal dialysis catheter placement. Tech Vasc Interv Radiol. 2017;20:75-81.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of an improved percutaneous peritoneal dialysis catheter placement technique in urgent-start peritoneal dialysis patients: A retrospective cohort study. Ann Palliat Med. 2022;11:3455-63.
- [CrossRef] [PubMed] [Google Scholar]
- Complications of percutaneous peritoneal dialysis catheter. Semin Intervent Radiol. 2022;39:40-6.
- [CrossRef] [PubMed] [Google Scholar]
- ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 update (adults) Perit Dial Int. 2021;41:15-31.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided percutaneous peritoneal dialysis catheter insertion using multifunctional bladder paracentesis trocar: A modified percutaneous PD catheter placement technique. Semin Dial. 2020;33:133-9.
- [PubMed] [Google Scholar]
- Surgical versus percutaneous catheter placement for peritoneal dialysis: An updated systematic review and meta-analysis. J Nephrol. 2021;34:1681-96.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Peritoneal dialysis in the modern era. Perit Dial Int. 2023;43:301-14.
- [CrossRef] [PubMed] [Google Scholar]
- Urgent-start dialysis: Comparison of complications and outcomes between peritoneal dialysis and haemodialysis. Perit Dial Int. 2021;41:244-52.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative analysis of percutaneous and open surgical techniques for peritoneal catheter placement. Perit Dial Int. 2012;32:628-35.
- [CrossRef] [PubMed] [Google Scholar]







