Translate this page into:
Utility of POCUS (Point of Care Ultrasound) in Renal Transplantation
Corresponding author: Edwin M Fernando, Department of Nephrology, Government Stanley Medical College and Hospital, Chennai, Tamil Nadu, India. E-mail: nephroeddy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Fernando EM, Balasubramaniam S. Utility of POCUS (Point of Care Ultrasound) in Renal Transplantation. Indian J Nephrol. doi: 10.25259/IJN_245_2024
Abstract
Point of care ultrasound (POCUS) is gaining wide recognition in its bedside applications. The day-to-day practice of nephrology requires several ultrasonographic parameters for diagnosis. Hence, familiarity with basics of renal ultrasound imaging is becoming a necessary skill for every nephrologist. This review provides an overview of the normal and abnormal findings in a graft kidney and its environment throughout graft survival and after its failure. The correlative understanding of the clinical features with image findings provides the greatest advantage in applying POCUS at the bedside.
Keywords
POCUS
Renal transplant
Graft kidney
Acute rejection
Acute tubular injury
Introduction
The basics of clinical examination have been inspection, palpation, percussion, and auscultation since the nineteenth century. With the advances in diagnostic modalities, ultrasound imaging has taken a crucial place, with its bedside availability, non-invasive nature, rapid imaging, affordability, and no radiation risks. Hence, ultrasound is propounded as the fifth step of essential clinical examination under “POCUS,” point of care ultrasound.1
Understanding image acquisition and interpretation is the basic requirement for practicing POCUS. POCUS aims to clarify simple clinical queries to clinch the diagnosis faster and to perform simple and basic bedside interventions.
In the transplant situation, POCUS is used in conditions of reduced urine output and suboptimal graft function, to assess for possible perigraft collections, fluid overload, and so on. It is utilized to perform guided drainage of collections and obtain tissue samples. Color Doppler application adds to the information and plan a prompt intervention or reassure the patient.
Basic ultrasound physics, modes, and instrumentation
Audible sound has a frequency of 20–20,000 Hz. Frequencies above this range are called ultrasound. Medical ultrasound imaging uses sound waves of 1–20 megahertz (MHz), which is significantly above the limit of human hearing.2
Wavelength and frequency: Sound requires a matter or a medium for transmission. The propagation of sound waves is in the form of a sine wave [Figure 1a], causing compression and rarefaction of the particles of the medium.
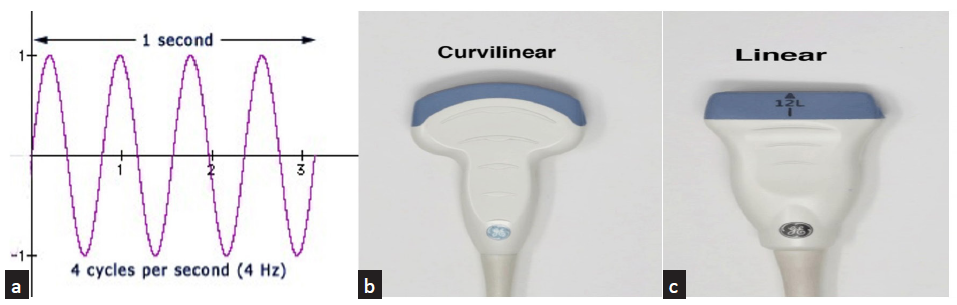
- (a) Sinusoidal sound wave; (b) convex probe: frequency range of 2–5 MHz; (c) linear probe: frequency range of 7–20 MHz.
The distance between peaks of two consecutive sine waves is one cycle, equal to the wavelength. In diagnostic ultrasound, the wavelength is in the order of millimeters. The number of cycles that occur in 1s is the frequency of a given wave.
Piezoelectric property and its application in the ultrasound probe: The source of ultrasound in the scanner is Lead Zirconate Titanate amalgam, a crystal with piezoelectric property which bestows the ability of these crystals to vibrate and produce sound waves when an electrical voltage is applied and vice versa. The probes also receive the echoes returning from the organs and convert them into electrical energy, which is transformed into images with different shades of brightness on a grayscale.3
The various modes of ultrasound display4 are as follows:
a. A-mode, also known as “Amplitude modulation,” is a display of the sound waves returning from the organ in spikes plotted on a graph as a function of depth. It is commonly used in ophthalmology and to treat tumors or calculi with focused ultrasound waves.
b. B-mode or the “brightness mode” denotes a regular 2-dimensional grayscale display used in clinical practice.
c. In M-mode or the “motion mode,” a series of images obtained in B-mode is displayed, which aids in the visualization and measurement of the movements of the insonated structure.
d. The color Doppler mode applies the “Doppler effect” to assess movement toward or away from the probe. These movements are given different colors like red, blue, green, yellow, etc. This model is applied to study the motion of blood or parts of the heart.
Display of the Doppler information may be done in one or more of the following ways:
Color Doppler: color-coded image with directional information
Power Doppler: color-coded image with no directional information but very sensitive to minimal or slow flow5
Spectral Doppler: graphic representation of flow
Probes have footprints of different sizes and shapes capable of delivering and receiving ultrasound waves of a predetermined range. The curvilinear probes [Figure 1b] with a frequency range of 2–5 MHz and linear probe with a frequency range of 7–20 MHz [Figure 1c] are used to study the graft kidney. The higher the frequency of the ultrasound waves, the lesser the penetration. Hence, the curvilinear probes are used for abdominal organs, and the linear high-frequency probes are used for structures closer to the skin, such as the arteriovenous fistula and dialysis catheters. As the graft kidney is closer to the anterior abdominal wall in the iliac fossa, the linear probe may also be used to get information from the graft.
Brightness of various tissues
The interaction between the ultrasound waves and the tissue determines the appearance of the various insonated tissues. Tissues or substances that allow the transmission of ultrasound waves appear anechoic [Figure 2a]. Irregular surfaces, fluids with echogenic contents [Figure 2b], and solid organs with nonuniform density scatter the ultrasound waves, and some of these reach the transducer to produce an image of varying brightness. Tissue interfaces that reflect sound waves appear bright or hyperechoic with posterior acoustic shadowing [Figure 2c].
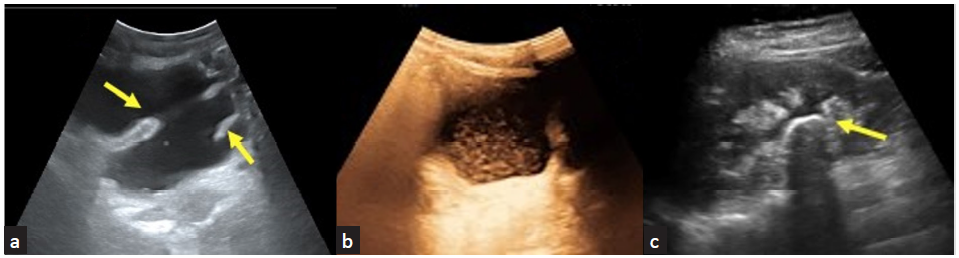
- (a) Anechoic clear urine in a renal recipient with neurogenic bladder. Trabeculated walls of the bladder seen (yellow arrows); (b) echogenic contents in the urine in cystitis; (c) curvilinear renal pelvic calculus (yellow arrow).
Evaluation of Transplant Kidney by Ultrasound
POCUS in renal transplantation could include evaluating the recipient’s aorta and iliac vessels, intraoperative evaluation, and postoperative assessment of the graft, its vasculature, and environment. A basic understanding of the graft’s appearance, placement, and anastomosis of the graft vasculature is essential to performing the ultrasound examination and interpreting the images obtained. Intraoperative images or diagrams of the graft and its anastomosis in the surgical notes aid in image interpretation.
Placement of the Graft Kidney
The common practice is to place the donor’s left kidney in the recipient’s right iliac fossa [Figure 3a].6 This enables vascular anastomosis between the vessels without causing traction. Commonly performed arterial anastomosis is end-to-side anastomosis with the external iliac artery or end-to-end to the recipient’s internal iliac artery. Venous anastomosis is usually end-to-side to the recipient’s external iliac vein. In deceased donors, a patch of the donor aorta is removed along with the single or multiple renal arteries, and this patch is anastomosed end-to-side with the recipient’s external iliac artery, thereby avoiding multiple anastomoses in the latter case. The ureter of the donor is sutured at the dome of the bladder, and this is called neocystoureterostomy.2
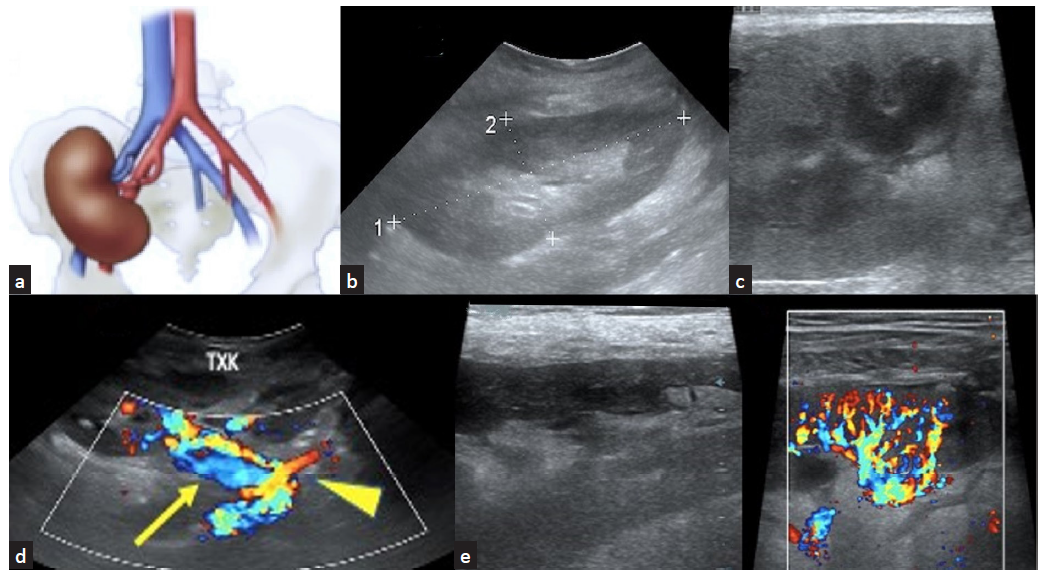
- (a) Graft kidney placed in RIF depicting the nature of anastomosis of the renal artery and vein; (b) normal graft kidney in RIF; (c) renal cortex and medulla; (d) normal graft vessels (yellow arrow) including anastomotic site (yellow arrowhead) are visualized well; (e) graft vascularity including arcuate vessels seen on color Doppler study.
In situations where the donor is an adult and the recipient is a pediatric patient, or if the space in the recipient’s right iliac fossa is a constraint, the graft is placed in the retroperitoneal space of the abdomen. In such a scenario, vascular anastomosis is to the recipient’s aorta and inferior vena cava (IVC).
Evaluation of the Graft Kidney by Ultrasound
Evaluation of the graft includes anatomical assessment including the renal size, cortical echoes, and status of the pelvicalyceal system and renal vasculature, position of stent, if present, assessment of perigraft areas for collections and as an aid to perform guided biopsy and to assess its attendant complications.
Baseline evaluation includes assessment with the curvilinear and linear probes. Renal size, corticomedullary differentiation, vascularity, perinephric collections, ureter, vesicoureteric anastomotic site, double J ureteric stent (DJ stent), if present, iliac vessels, and IVC are assessed. The initial baseline assessment is done within 48 h post surgery. However, earlier evaluation of the graft is warranted if indicated.
Normal Appearance of the Graft Kidney and its Vasculature
Corticomedullary differentiation is seen well, as the graft is closer to the anterior abdominal wall than the native kidney [Figure 3b and 3c]. The pelvis of the graft can be dilated due to back pressure from the urinary bladder due to the lack of the natural antireflux mechanism at the vesicoureteric junction, a single kidney handling the urine output, and a denervated donor ureter.
Vessels are visible well due to the proximity to the skin. Color Doppler helps to visualize the anastomotic site and the main and intrarenal vessels [Figure 3d and 3e]. Spectral Doppler shows a steep systolic peak and a sloping diastolic flow until the following systolic flow takes over.7 The peak systolic velocity (PSV) and the end diastolic velocity (EDV) are useful to calculate the resistivity index (RI) which is a measure of resistance to blood flow.
RI = [PSV – EDV]/PSV
The resistivity index of the normal intragraft vasculature is less than 0.8.
The resistivity index is an indicator of vascular resistance and compliance. It is influenced not only by renal histologic factors such as ATI, rejection, cortical necrosis, and parenchymal edema due to RVT but also by many factors such as the proximal and distal resistance, atheromatous changes in the vessel wall, mean arterial pressure, pulse rate, left ventricular status, age of the patient, and pressure applied on the probe while examining surrounding parenchyma.8,9 Though there are many nonrenal factors affecting the renal RI, it is helpful to predict graft dysfunction in the initial postoperative weeks. It is recommended to assess the RI of graft on an annual basis since this will serve as a comparison in the event of future graft dysfunction.
Power Doppler is an additional tool to assess the perfusion of the graft kidney. DJ stent, if present, is seen as a pair of parallel hyperechoic linear focus [Figure 4].
- Double J (DJ) stent (yellow arrows) seen in the (a) graft renal pelvis, (b) ureter and in the (c) urinary bladder.
POCUS for Assessment of Complications
Routine bedside protocol-based assessment of the graft and its environment helps in the early identification of complications.
Parenchymal complications
These include acute tubular injury (ATI), rejection, adverse effects of drugs on the renal parenchyma, and infection. POCUS helps to identify and monitor these complications and guides in obtaining a tissue sample.
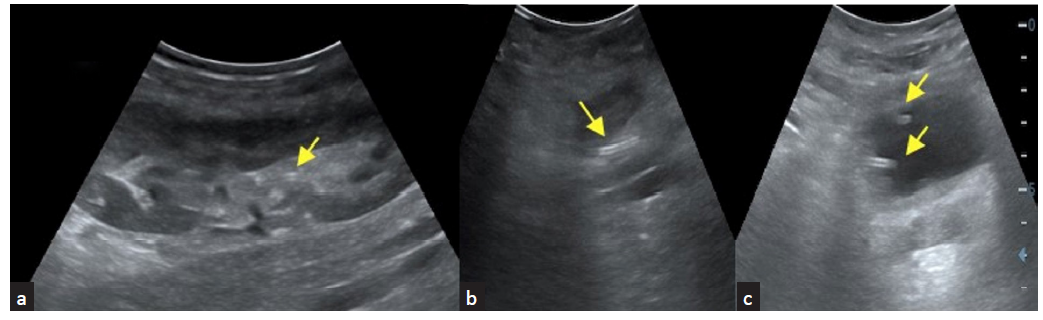
Acute tubular injury (ATI) in patients with prolonged ischemia time who underwent deceased donor allograft transplant shown in Figure 5a-b. ATI due to prolonged ischemia time or drug toxicity and acute rejections show similar non specific findings like parenchymal edema with increased intrarenal vascular resistance.10 Figure 5c shows color doppler image of graft kidney with acute cell mediated rejection.
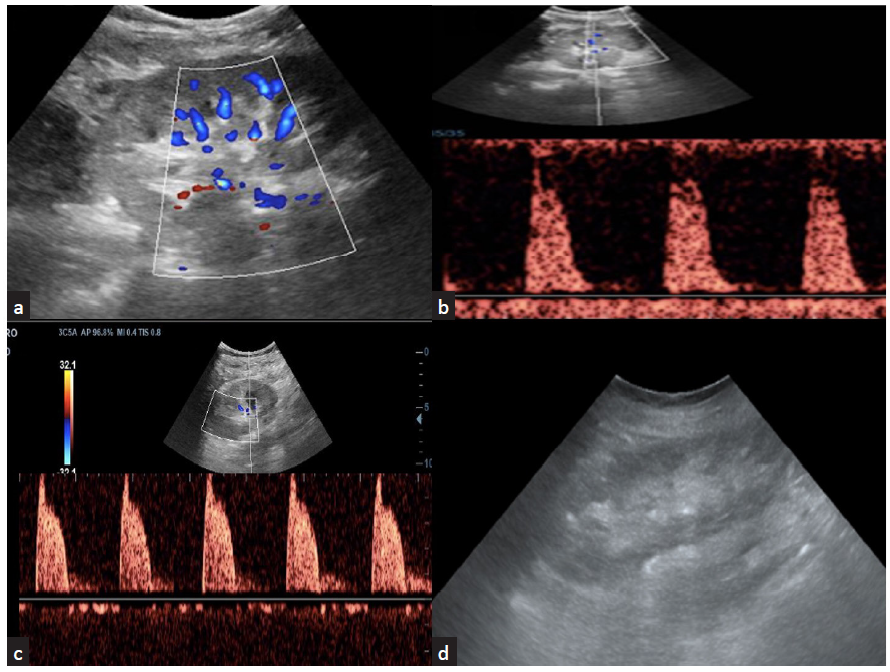
- (a) Acute tubular injury in an allograft with prolonged ischemia time with color Doppler images show sparse vascularity; (b) spectral Doppler shows increased vascular resistance; (c) color Doppler imaging shows increased vascular resistance seen in a graft kidney with acute cell-mediated rejection; (d) chronic allograft nephropathy shows cortical thinning with increased echogenicity of the cortex.
The spectrum of parenchymal complications include acute tubular injury (ATI), rejection, chronic allograft nephropathy, and infections”. Figure 5 and Supplementary Figures 1 and 2 show representative images depicting these abnormalities on USG.
Pyelonephritis, papillary necrosis, pyonephrosis, abscess [Supplementary Figure 1a], and fungal balls may be caused by infection. Thickening of urothelial lining due to edema [Supplementary Figure 1b] and echogenic collections in the collecting system are the sonographic findings in pyelonephritis and pyonephrosis, respectively.
Reactivation of JC polyomavirus resembles acute renal tubular necrosis with raised vascular resistance and may cause thickening of the urothelium. Infection in other organs like the prostate gland may also be encountered [Supplementary Figure 1c]. Failed graft if left insitu may appear as shrunken kidney infiltrated by fat or as shrunken kidney with dystropic calcification [Supplementary Figure 2].
Vascular complications
The early detection of vascular complications helps intervene and institute appropriate management. Color Doppler helps to identify the vascular complications at the bedside. Knowledge of the number of arteries and veins and the nature of anastomosis is important to obtain the best possible interpretation of the images. Important vascular complications include renal artery stenosis (RAS), renal vein thrombosis (RVT), pseudoaneurysm (PA), and arteriovenous fistula (AV fistula).
Renal artery stenosis: The common site of stenosis is at the anastomosis. End-to-end anastomosis of graft artery to the internal iliac artery carries a three-fold increased risk of stenosis when compared to end-to-side anastomosis with external iliac artery. Criteria for diagnosing renal artery stenosis include peak systolic velocity greater than 200 cm/s at the stenotic site, velocity at the immediate post-stenotic site is at least twice higher than that in the pre-stenotic site and tardus parvus pattern of spectrum in the segmental arteries with an acceleration time greater than 70 ms and an acceleration index less than 300 cm/s2.
Infarction of graft: Infarction of the graft, usually seen in the immediate postoperative period, may occur due to severe rejection, and arterial occlusion, kinking, or intimal tear with flap leading to arterial thrombosis. USG with color Doppler study shows the absence of arterial and venous flow in patients with complete occlusion. In patients with segmental or smaller arterial occlusion, wedge-shaped hypoechoic areas may be seen in the parenchyma.7
Renal vein thrombosis: Renal vein thrombosis (RVT) is seen in less than 5% of renal transplant recipients. USG shows edematous graft, increased resistance in the graft arteries, and thrombus in the renal vein [Supplementary Figure 3a and 3b]. It is important to assess whether there is an extension of the thrombus into the common or external iliac veins [Supplementary Figure 3c]. As with arterial occlusion, early recognition helps salvage graft.
Arteriovenous (AV) fistula and pseudoaneurysm: These can be a sequel to a biopsy of the graft. An arteriovenous fistula appears as a turbulent flow. A pseudoaneurysm appears as a focal vascular dilatation or an anechoic lesion that shows swirling flow within the lumen termed the “Yin–Yang” phenomenon [Supplementary Figure 3d and 3e].
Collecting System Complications
These complications include urine leaks and obstructions anywhere from the renal pelvis to the urinary bladder.
Urine leak and urinoma
Uninfected urinary collections appear anechoic and are seen between the graft and the urinary bladder. POCUS has a minimal role in identifying site of urine leakage hence additional investigations like CT urogram, renal scintigram may be required. However POCUS can be used to guide aspiration of the fluid to check the creatinine levels and compare with that of patient’s serum and urine creatinine levels.
Urinary obstruction
Urinary obstruction is seen in less than 2% of recipients. A distal third of the ureter is more likely to be involved due to the relatively compromised blood supply. USG shows dilatation of the collecting system and ureter [Supplementary Figure 4]. USG also helps assess the site and cause of obstruction and guides in percutaneous nephrostomy.
Calculus in graft kidney
Calculus in a graft may be seen in approximately 2% of the recipients. USG findings are similar to those of calculus in the native kidneys and include a curvilinear echogenic focus [Supplementary Figure 5] with posterior acoustic shadowing. Color Doppler study shows twinkling artefacts.
Perigraft collections
Perigraft collections are seen in approximately half of the patients receiving a graft. In the immediate postoperative period, hematoma, seroma, and urinoma are the commonly seen collections. Lymphoceles are commonly seen 4–8 weeks post-surgery. POCUS helps to assess and follow up on the size of the lymphocele. POCUS also helps to guide diagnostic and therapeutic aspiration.
Hematoma: On USG, the echogenicity of the hematoma depends on the duration of the hemorrhage, the presence of clots, and the evolution of the hemorrhage. Hyperacute hematomas appear anechoic. Acute hematomas appear to have mixed echoes with multiple echogenic foci [Supplementary Figure 6a]. Later, clots develop, adding heterogeneity to the imaging appearance. Chronic hematomas appear as anechoic collections with thin septations. POCUS helps to monitor the size, appearance, and mass effect, if any, on the graft. Intervention is warranted only if there is a continuous increase in size with a fall in hemoglobin levels or if there is a significant mass effect on the graft. [Supplementary Figure 6b].
Urinoma: USG shows anechoic collection with or without septations [Supplementary Figure 6c].11-13
Lymphocele: Lymphoceles are anechoic, septated collections [Supplementary Figure 6d] that tend to collect due to surgical disruption of focal lymphatic drainage. They tend to collect between the graft and the urinary bladder, sometimes causing mass effects on the ureter or the graft vessels. POCUS helps to guide diagnostic and therapeutic aspiration. USG-guided aspiration helps relieve the mass effect.
Abscess: On USG, the collection appears thick-walled with internal echoes and septations. USG guidance for diagnostic and therapeutic purposes is routinely employed.12,13
Contrast-enhanced USG (CEUS): Vascular, parenchymal and collecting system including ureter and bladder complications14,15 that may require contrast enhanced CT or MRI evaluation may be subjected CEUS since the contrast agents used here are safe to the renal parenchyma. The contrast agents can be administered intravenously or into the cavities. These agents are microbubbles of a gas like sulfur hexafluoride stabilized by a outer layer of protein or lipid. The gas bubbles gives the birefringence on application of US to generate the contrast enhanced images.14 The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines and recommendations provide the necessary guidelines on the use of ultrasound-contrast agents.15,16
POCUS also includes the assessment of IVC diameter [Supplementary Figure 7] to determine the fluid status of the recipient and examination of the live donor for possible collections in the nephrectomy bed [Supplementary Figure 8a] and other complications.17 POCUS also finds application to assess the graft along with its environment following a biopsy [Supplementary Figure 8b], to rule out the attendant complications.18
NephroPOCUS.com, a nephrologist-designed website recognized by the American Society of Nephrology as an innovative teaching tool, is used as a primary resource to supplement routine lectures and write-ups.
POCUS is an invaluable addition to the armamentarium of a nephrologist. It provides insights into pathophysiology at the bedside, enhances patient care and satisfaction, and has the potential to reduce the healthcare cost burden. It is imperative to recognize that while POCUS may alleviate the need for further investigations in some cases, it is not always a replacement for formal ultrasonography. Ultrasonography is an operator-dependent procedure. Hence, the services of a radiologist should be sought in case any doubts about the diagnosis or findings arise. As with any other skill, the use of POCUS by the nephrologist requires hands-on training, skill, dedication, and commitment.
Conflicts of interest
There are no conflicts of interest.
References
- Time to add a fifth pillar to bedside physical examination: inspection, palpation, percussion, auscultation, and insonation. JAMA cardiology.. 2018;3(4):346-50.
- [CrossRef] [PubMed] [Google Scholar]
- Application of ultrasound in medicine. Acta Informatica Medica.. 2011;19(3):168.
- [CrossRef] [Google Scholar]
- Point of care renal ultrasonography for the busy nephrologist: a pictorial review. World Journal of Nephrology.. 2019;8(3):44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The nephrologist as an ultrasonographer. Advances in Chronic Kidney Disease.. 2020;27(3):243-52.
- [CrossRef] [PubMed] [Google Scholar]
- Current progress in Nephrology (3rd ed). Mumbai: Tree life Media; 2022. p. :76-94.
- Ultrasonography in acute kidney injury. POCUS journal.. 2022;7(Kidney):35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Renal relevant radiology: use of ultrasound in kidney disease and nephrology procedures. Clinical Journal of the American Society of Nephrology.. 2014;9(2):373-81.
- [Google Scholar]
- Value and limitations of sonography in kidney transplant recipients with special attention to the resistive index–An update. Frontiers in Nephrology.. 2022;2:997839.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Usefulness of renal ultrasonography for assessment of severity and course of acute tubular necrosis. Journal of clinical ultrasound.. 1984;12(3):135-9.
- [CrossRef] [Google Scholar]
- Radiological imaging in renal transplantation. Acta Clinica Croatica.. 2018;57(4):694.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ultrasonographic features of kidney transplants and their complications: an imaging review. International Scholarly Research Notices.. 2013;2013(1):480862.
- [CrossRef] [Google Scholar]
- Imaging of renal transplant complications throughout the life of the allograft: comprehensive multimodality review. Radiographics.. 2019;39(5):1327-55.
- [CrossRef] [PubMed] [Google Scholar]
- Contrast-enhanced US in renal transplant complications: Overview and imaging features. RadioGraphics.. 2024;44(6):e230182.
- [CrossRef] [PubMed] [Google Scholar]
- A novel simple noninvasive index to predict renal transplant acute rejection by contrast-enhanced ultrasonography. Transplantation.. 2015;99(3):636-41.
- [CrossRef] [PubMed] [Google Scholar]
- The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall in der Medizin-European Journal of Ultrasound.. 2012;33(01):33-59.
- [Google Scholar]
- Inferior vena cava ultrasound and other techniques for assessment of intravascular and extravascular volume: an update. Clinical Kidney Journal.. 2023;16(11):1861-77.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- New ultrasound techniques promise further advances in AKI and CKD. Journal of the American Society of Nephrology.. 2017;28(12):3452-60.
- [CrossRef] [Google Scholar]








