Translate this page into:
A Challenging Case of Primary Membranous Nephropathy with Renal Cell Carcinoma – Double Whammy
Corresponding author: Shyam B. Bansal, Department of Nephrology and Renal Transplant Medicine, Medanta The Medicity, Gurugram, Haryana, India. E-mail: drshyambansal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Manhas N, Bansal SB, Rana AS, Rana A, Sethi SK, Mahapatra A. A Challenging Case of Primary Membranous Nephropathy with Renal Cell Carcinoma – Double Whammy. Indian J Nephrol. 2024;34:657-8. doi: 10.25259/ijn_492_23
Abstract
Membranous nephropathy is the most common cause of nephrotic syndrome in adults. It can be associated with malignancy especially in elderly patients, however; sometimes it is difficult to distinguish. Hereby we present an interesting case of primary membranous nephropathy in a patient with renal cell carcinoma.
Keywords
Membranous nephropathy
renal cell carcinoma
malignancy
phospholipase A2 Receptor antibody
Introduction
Membranous nephropathy (MN) is the most common cause of nephrotic syndrome in adults worldwide. Most cases of MN are caused by antibodies to phospholipase receptor A2 (70%).1 Approximately 20%–30% of MN is related to malignancy, especially in people over the age of 50 years. It is, therefore, necessary to look for an occult malignancy in these patients. Hereby, we describe a challenging case of primary MN in a patient with renal cell carcinoma (RCC).
Case Report
A 57-year-old male with long-standing hypertension, hypothyroidism, and coronary artery disease was found to have a solid heteroechoic exophytic lesion measuring 2.4 × 2.2 cm in the interpolar region of left kidney with internal vascularity on ultrasound during routine evaluation in 2016 [Figure 1]. He was advised to follow-up with the treating urologist.
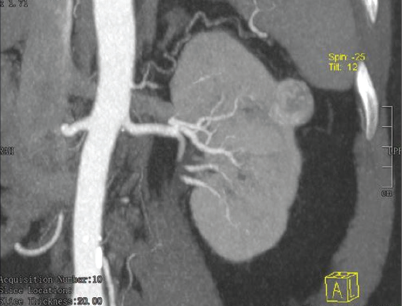
- CT urography revealing exophytic hypodense lesion of size 22 × 21 × 22 mm arising from the lateral cortex at the upper pole of the left kidney, showing heterogenous arterial enhancement. CT = computed tomography
In 2020, he developed bilateral symmetrical pedal edema, which progressed to anasarca. On evaluation, his serum creatinine was 1 mg/dl; however, his urine routine revealed 2+ protein and urine protein/creatinine ratio of 1 g/g. He was advised to either remain on surveillance or opt for surgical intervention. Patient refused surgery and was kept on symptomatic treatment for edema.
In February 2022, he presented with worsening edema. Laboratory evaluation revealed serum creatinine of 1.5 mg/ dl, urine albumin/creatinine ratio of 8 g/g with serum albumin of 1.9 g/dl [Table 1]. The serum anti-PLA2R antibody titre was 794 RU/ml. Repeat computed tomography (CT) urography revealed the same exophytic lesion with heterogenous arterial enhancement with partial washout of contrast in the delayed phase and no gross change in size.
| Before surgery | 3 months on ponticelli regimen | 6 months on ponticelli regimen | 1 year on post-ponticelli | Current status | |
|---|---|---|---|---|---|
| Creatinine (mg/dl) | 3 | 1.9 | 1.7 | 1.2 | 1.3 |
| Albumin (g/dl) | 1.6 | 2.6 | 2.55 | 3.8 | 4.5 |
| Cholesterol/triglyceride/HDL | 168/289/41 | 123/130/46 | |||
| Urine protein creatinine ratio (spot) g/g | 8 | 4.2 | 2.5 | 0.45 | 0.80 |
| Serum PLA2RAb level (RU/ml) | 794 RU/mL | Negative (<2 RU/ml) | Negative (<2 RU/ml) |
HDL: High-density lipoprotein, PLA2RAb: Phospholipase A2 receptor
He underwent partial nephrectomy in March 2022 [Figure 2]. Histology confirmed clear cell renal carcinoma pT1a Nx, resection margin free. PLA2R was negative in the tumor tissue. Sections from the adjoining nontumorous portion of the kidney revealed diffuse uniform basement membrane thickening with mild, variable mesangial matrix expansion and no increase in mesangial cellularity. Immunofluorescence revealed IgG4- 3+ and IgG 1- 1+ along the capillary loops and kappa and lambda both 3+ granular along the capillary loops. These were suggestive of MN, and staining for PLA2R was positive in the non-neoplastic region.
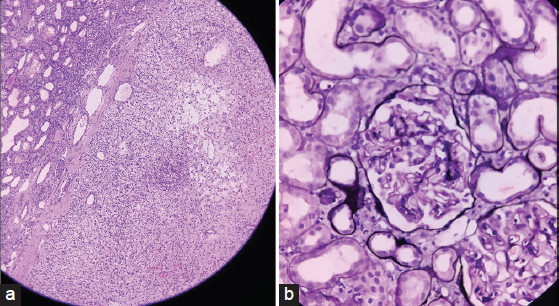
- (a) Tumor reveals typical patterns including nests with delicate interconnecting vessels. The cytoplasm of tumor cells is clear and eosinophilic (H and E stain, 10×). (b) Light microscopy-Diffuse uniform basement membrane thickening with variable mesangial matrix expansion in the nontumorous portion (methenamine silver stain, 100×). H and E = hematoxylin and eosin
There was no improvement in his proteinuria after 1 month. In view of severe proteinuria, rise in serum creatinine to 2 mg/dl and hypoalbuminemia (1.6 g/dl), he was started on the modified Ponticelli regimen. His proteinuria gradually reduced and kidney function improved. On follow-up, 1 year after starting the modified Ponticelli regimen, his proteinuria had reduced to 1.2 g/day and the anti-PLA2R antibody was negative. Patient is currently doing well on antihypertensives, antiplatelet, and statin.
Discussion
The association between nephrotic syndrome and neoplasia is well known.2 Glomerular disease can develop in patients with malignancy due to deposition of circulating tumor antigen–antibody complexes or in situ immune complex formation. However, the search for a specific antigen–antibody mechanism has only been partly successful.
The rare association of renal cell carcinoma with MN could be explained by the lesser tendency for tumor antigen release to occur from RCC than from other cancer cells.3 RCC may introduce cancer-related abnormal T cells less often.4 Another hypothesis involves a masking effect of progressed RCC by the clinical symptoms of nephrotic syndrome.5
This raises the question of whether this is secondary MN associated with malignancy and coincidentally discovered anti-PLA2R autoantibodies or primary MN due to anti-PLA2R autoantibodies with coincidentally active malignancy. MN with positive anti-PLA2R autoantibodies and IgG4-predominant staining may be more physiologically related to primary MN, with their associated disease process (neoplasm, autoimmune, and viral hepatitis) being coincidental.6 There is no definitive evidence to support malignancy-mediated production of anti-PLA2R or cross-reactivity with tumor antigens.
This is a rare case of biopsy-proven PLA2R-associated MN in the setting of renal cell carcinoma. Anti-PLA2R seropositivity should not be considered sufficient to refrain from evaluating for other secondary etiologies, and age-appropriate cancer screening should be pursued in all patients with a diagnosis of MN. The association between malignancy and anti-PLA2R positivity merits further investigation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The association of cancer and the nephrotic syndrome. Ann Intern Med. 1966;64:41-51.
- [CrossRef] [PubMed] [Google Scholar]
- Membranous nephropathy associated with renal cell carcinoma. Am J Kidney Dis. 1993;22:352-2.
- [CrossRef] [PubMed] [Google Scholar]
- Minimal-change glomerulopathy and carcinoma: Report of two cases and review of the literature. Am J Nephrol. 1993;13:69-72.
- [CrossRef] [PubMed] [Google Scholar]
- Membranous nephropathy complicated by renal cell carcinoma. J Clin Exp Nephrol. 2004;8:59-62.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Modern Pathol. 2013;26:709-15.
- [CrossRef] [PubMed] [Google Scholar]







