Translate this page into:
A Review of Residual Kidney Function in Peritoneal Dialysis Patients
Address for correspondence: Dr. Ibrahim Mohammed Alrowiyti, Department of Nephrology, University Health Network, 190 Elizabeth St, Toronto, Ontario M5G 2C4, Canada. E-mail: Ibrahim.alrowiyti@mail.utoronto.ca
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Residual kidney function (RKF) has been associated with better survival, less morbidity, and improved quality of life in peritoneal dialysis (PD) patients. Since higher peritoneal clearance does not lead to better outcomes, more emphasis should be put on preserving kidney function. Many other benefits have been reported, including better volume and blood pressure control, better nutritional status, lower rates of PD peritonitis, preserved erythropoietin and vitamin D production, middle molecule clearance, lower Left Ventricular Hypertrophy, and better serum phosphate level. The most practical method of assessing RKF is the mean of 24-h urinary urea and creatinine clearance. Incremental PD prescription is an ideal option to supplement RKF in PD patients, which also offers more flexibility to the patient and, possibly, improved adherence. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers should be used when possible in PD patients to preserve RKF. Loop diuretics are underutilized in PD patients despite providing an additional means of maintaining fluid balance and reducing the need for higher glucose-containing PD solutions. In this paper, we outline the importance of RKF in PD patients and the different strategies for its preservation.
Keywords
Incremental peritoneal dialysis
peritoneal dialysis
residual kidney function
Importance
The importance of residual kidney function (RKF) in peritoneal dialysis (PD) patients has been highlighted in multiple clinical trials. Although the CANUSA study showed better survival with greater total (peritoneal and kidney) small molecule clearance as measured by weekly Kt/v and creatinine clearance in PD patients,[1] this association was not found to be significant in many other studies. RKF was shown to have a favorable outcome in PD patients in terms of improved mortality, morbidity, and quality of life.[234567] In our reanalysis of the CANUSA study, which included 680 patients on PD, it was found that a 5-L greater weekly glomerular filtration rate (GFR) was associated with a 12% decrease in relative risk of death (95% confidence interval [CI], 0.83 to 0.94). There was no association between the same increase in peritoneal creatinine clearance and RR of death (RR: 1.00; 95% CI: 0.90 to 1.11). Also, for each increase of 250 ml of urine per day, there was a 36% decrease in RR of death (RR: 0.64; 95% CI: 0.51 to 0.80). Neither net peritoneal ultrafiltration nor total fluid removal per 24 h was associated with better survival.[2] Termorshuizen et al.[6] also reported better survival with higher RKF, but unlike the CANUSA reanalysis, the authors could not confirm the effect of urine output independent of residual GFR. Interestingly, the effect of RKF becomes less pronounced after adjusting for hemoglobin concentration, suggesting that a higher hemoglobin concentration could have a protective effect against RKF deterioration. The favorable effects of RKF may be related to better volume control, large and middle molecule clearance, or reduced inflammation.[28910] In a retrospective study of 207 patients who started on PD, blood pressure control was worse with declining RKF.[11] Other potential benefits of higher RKF include improved nutritional status,[11213] reduced left ventricular hypertrophy,[1415] and lower levels of serum phosphate and uric acid. In addition, lower rates of PD peritonitis have been associated with RKF,[161718] although the mechanism is still unclear. Figure 1 illustrates the major benefits of RKF in PD patients.

- Benefits of residual kidney function in peritoneal dialysis patients
Definition
Although there is no universal definition of RKF, it can be viewed as the remaining GFR from the native kidneys in a patient with end stage kidney disease (ESKD). Anephric can be defined as either a GFR <1 ml/min/1.73M² or 24-h urine volume of <100 ml or creatinine clearance <1 ml/min.[919] As stated by the National Kidney Foundation-Kidney Disease Outcome Quality Initiative (NKF-KDOQI), urine output >100 ml/day should be considered significant. Although there is no good evidence, residual urine volumes of less than 100 ml/day may also be important.
How to measure RKF?
The most widely used method for RKF measurement is the mean 24-h urea and creatinine clearance as recommended by the National Kidney Foundation-Kidney Disease Outcome Quality Initiative (NKF-KDOQI and other reports.[2021] This method remains easier and more applicable in the clinical setting than other tests such as inulin, iohexol, ethylenediaminetetraacetic acid, and iothalamate. The ideal marker must be freely filtered by the glomeruli and not secreted by the tubules, and without any interference from PD or hemodialysis (HD) clearance.[22] van olden et al.[21] reported improved accuracy for GFR estimation from creatinine clearance that approximates inulin with the administration of cimetidine. However, this method is more suitable for research setting, while the mean clearance of urea and creatinine can be useful in a clinical sitting. Low-molecular-weight proteins such as cystatin C, β2-microglobulin (B2M), and β-trace protein (BTP) have been studied to estimate RKF in the dialysis population.[2324] Cystatin C formula has been reported to be more accurate for estimation of residual GFR in dialysis patients when 24-h urine is not available or unreliable.[2526] Cystatin C has its own drawbacks, as it may overestimate GFR and its levels can be affected by age, diabetes mellitus, higher white cell count, low albumin, high C-reactive protein (CRP), high body mass index (BMI), and female gender.[27] Other markers that have been reported include C-terminal agrin fragment,[28] p-cresyl sulfate, and indoxyl sulfate.[29] Although these tests seem promising and might be advantageous compared to the more cumbersome 24-h urine collection, none of these new markers has been validated to use in the clinical setting. The 24-h urine collection can be cumbersome for the patient and is prone to errors, yet it remains the recommended and the most appropriate method of estimating RKF in the clinical sitting. If the patient refuses to perform a 24-h collection, and if the serum creatinine concentration is unchanged in the face of an unchanged PD prescription, we assume that the renal creatinine clearance is also unchanged.
Factors affecting RKF
In general, PD offers better protection to RKF than HD, probably due to gentle ultrafiltration over longer hours, which could mean better hemodynamics and less fluctuation in blood pressure.[3031] Studies have demonstrated a slower rate of decline in RKF in patients who start on PD compared to the predialysis period.[323334] Moist et al.[30] in a large prospective study that included 1032 patients on PD and 811 patients on HD, reported a 65% lower risk of RKF loss in PD patients compared to those on HD (adjusted odds ratio = 0.35, P: 0.001). Factors reported by Moist et al.[30] that were associated with loss of RKF included female sex, non-white race, history of diabetes, history of congestive heart failure, longer time to follow-up, and lower RKF at the start of dialysis. On the other hand, factors associated with better preservation of RKF included treatment with angiotensin converting enzyme (ACE) inhibitors, treatment with calcium channel blockers, higher GFR at baseline, and higher serum calcium levels. Moist et al.[30] did not find an association between the type of PD modality (automated peritoneal dialysis [APD] vs. continuous ambulatory peritoneal dialysis [CAPD]) and the rate of RKF decline. Such an association has been reported by other studies, wherein there was less RKF loss among those who were on CAPD versus APD. The gentler nature and the constant rate (over 24 h) of CAPD might provide an explanation to the slower decline in RKF compared to APD observed in those studies. However, the majority of those studies were small, single-center studies and did not take into account the effect of variation in APD prescription on RKF, and most of the patients were not treated with renin–angiotensin–aldosterone blockers. There is not enough evidence to conclude that CAPD is superior to APD for preserving RKF.[35] In a prospective single-center study, with 270 patients starting PD between January 1996 and December 2005, diabetes mellitus, higher (rather than lower) baseline residual GFR, hypotensive events, use of diuretics, and episodes of peritonitis were independently associated with faster decline of residual GFR.[36] The effect of diuretics on RKF loss remains controversial and was not reproduced in other studies.[3738]
Incremental PD prescription
Incremental PD is focused on prescribing the minimum possible amount of dialysis dose to achieve patient well-being. The initial total volume is usually less than 6 l/day. The dose is then gradually increased as RKF declines over time.[39] This approach is by no means intended for patients with insufficient RKF and clearance function; rather, it is intended to supplement the already existing RKF to achieve better removal of uremic toxins, better volume control, and, at the same time, provide the patient more flexibility and symptom relief. This practice allows for more individualized prescription dose rather than adhering to a rigid dose upon starting PD. The recommended delivered dose of total small solute clearance should be at least Kt/V of 1.7–2.0 per week from both kidney and peritoneal clearance.[140] Kt/V urea is not without limitations, as there is no evidence that small solute clearance is associated with better outcomes. Also, it does not take into account other uremic toxins or salt and water balance.[41] When adjusting the PD dose, the nephrologist should consider multiple factors such as the overall clinical condition of the patient, quality of life, volume status, and patient’s wishes, rather than relying exclusively on target Kt/V urea. An incremental PD prescription has many benefits including slower RKF decline, improved quality of life, reduced health-care costs, lower rates of peritonitis, fewer connections, less exposure to glucose with its degradation products, and less plastic waste and water consumption.[3942434445] Incremental PD may be advantageous to the patient, as it requires less effort and connection handling, which might result in less anxiety and stress, especially for new start patients, and might enhance adherence. Lower dwell volume might lead to fewer mechanical side effects such as back pain, abdominal fullness, and reflux.[39] It is unclear whether incremental PD lowers the risk of peritonitis. In theory, less handling and connections might lead to fewer peritonitis episodes. However, this hypothesis needs robust clinical trials to confirm the association. In a single-center, prospective, randomized controlled study by Yan et al.,[46] 139 incident PD patients were randomized to an incremental CAPD dose (three exchanges) versus full-dose CAPD (four exchanges). The incremental group had a lower peritonitis rate, but this did not reach statistical significance (13% vs. 26%, P = 0.06). In a recent retrospective study following 106 PD patients divided into two groups, one with incremental prescription and the other with full prescription, there was a significant decrease in the probability of developing two or more episodes of peritonitis (odds ratio [OR] 0.35, 95% CI: 0.14–0.87, P = 0.046) in the incremental group compared to the full prescription group.[47]
Taking into consideration the patient’s quality of life, social situation, and shared decision-making, we suggest prescribing incremental PD dose whenever possible for individuals who have significant RKF. The starting prescription can be CAPD with two exchanges during the day and one longer exchange at night. The night exchange typically consists of icodextrin due to its ability to stay for longer hours without losing its ultrafiltration properties. The fill volume can be 1.5 l for the day dwell and 1.0 l for the night dwell [Table 1]. Another alternative would be with nocturnal intermittent PD, usually three exchanges over 8 h, typically 1.5 l for each fill.[48] A single exchange of icodextrin, 2.0–2.5 l over 8–16 h, for patients with sufficient RKF is also a reasonable approach and can be sufficient for many patients[4449] [Table 1]. The single daily exchange might also have some added benefits such as reducing the burden on the patient and reducing touch contamination. However, patients who qualify for a single daily exchange must be selected carefully, as it requires sufficient RKF.
| No. of exchanges | Fill volume | Duration | Frequency | |
|---|---|---|---|---|
| CAPD | 1 exchange of icodextrin | 2.0 or 2.5 l | 8–16 h | Daily |
| 3 | 2 l | 4 h for each dwella | Daily or 6 days a week | |
| 4 | 1.5 l | 4 h for each dwella | Daily or 6 days a week | |
| NIPD | 3 | 1.5 l | 8–10 h | Daily or 6 days a week |
| 3 | 1.5 l | 6 h | Daily | |
| 3 | 2.0 l | 8–10 h | 5 nights a weak |
aOne longer exchange of icodextrin can be used along with two shorter dwells. CAPD=continuous automated peritoneal dialysis, NIPD=nocturnal intermittent peritoneal dialysis, PD=peritoneal dialysis
PD patients on an incremental prescription should be closely monitored, and regular follow-up should be the standard of care to ensure adequacy of the current prescription and that solute clearance and target volume control are met.[50] The NKF-KDOQI guidelines recommend in any PD patient with >100 ml/day of residual kidney volume, the residual kidney clearance should be considered as part of the patient’s total weekly solute clearance goal, and a 24-h urine collection for urine volume and solute clearance determinations should be obtained at a minimum of every 2 months. Another approach is to monitor the patient’s response, clinical well-being, volume status, and other symptomatology that might be related to inadequate dialysis, as well as the biochemical markers, without the need for a regular 24-h urine collection to measure adequacy.[48] Such an approach was suggested by the International Society of Peritoneal Dialysis (ISPD) guidelines.[51]
Biocompatible neutral pH PD solution
The idea behind a “biocompatible” PD solution revolves around preventing glucose degradation products (GDPs) that might result from prolonged storage and heat sterilization process. GDPs have been associated with kidney injury via increased levels of glycosylation end products. Johnson et al.,[52] in a multicenter, randomized controlled trial (RCT), found that the use of biocompatible solution in incident PD patients was not associated with a significantly slower GFR decline, but rather, it significantly delayed the time to anuria and reduced the risk of peritonitis. However, a Cochrane review in 2014[53] showed that the use of neutral pH solutions was associated with higher urine volumes up to 3 years of therapy duration (mean difference126.39 ml/day, 95% CI: 26.73 to 226.05). There was a trend toward better preservation of RKF in studies with more than 12 months follow-up (six studies, 360 patients: standardize mean difference 0.31, 95% CI: 0.10 to 0.52). Another Cochrane review in 2018,[54] which added six new studies to the previous review, concluded with high certainty that the use of biocompatible PD solution resulted in better preservation of RKF by a mean difference in GFR of 0.54 ml/min/1.73 m² (95% CI: 0.14 to 0.93). However, an important confounding factor is that neutral pH, low-GDP solutions result in a reduction in ultrafiltration compared to conventional PD solutions. The resulting (clinical or subclinical) volume overload could be the principal driver of increased urine output. So, these new solutions may not be intrinsically nephroprotective as is commonly believed.[55]
Icodextrin
Icodextrin is commonly used in PD for the longer dwell. Unlike the glucose-based solution which can be absorbed via diffusion across the peritoneal capillary, icodextrin is mainly absorbed by convection out of the peritoneal cavity via the lymphatic system. This results in relatively constant oncotic pressure, which leads to sustained ultrafiltration during the long dwell time.[56] In a multicenter, prospective, randomized controlled study, 100 patients were randomized to either one exchange of icodextrin for more than 8 h dwell time and two exchanges of 1.5% glucose-based biocompatible neutral pH solution or one exchange of 2.5% and two exchanges of 1.5% glucose-based biocompatible solution. The icodextrin group had slower decline in RKF and daily urine volume declined faster in the glucose solution-based group.[57] In a meta-analysis by Qi et al.[58] including nine RCTs, icodextrin was associated with greater net ultrafiltration with no difference in RKF loss compared to the other glucose-containing solutions. In a more recent Cochrane review, icodextrin was associated with better ultrafiltration without compromising RKF.[59] The mechanism of ultrafiltration without compromise in RKF is speculative and may be related to sustained osmotic diuresis of icodextrin metabolites or flux of fluid from the intracellular to extracellular compartment driven by the metabolites. In the light of current evidence, it is not unreasonable to use icodextrin when formulating a PD prescription as it offers an excellent means of sustained ultrafiltration with little impact of RKF in PD patients.
ACE inhibitors and angiotensin receptor blockers
The use of ACE inhibitors and angiotensin receptor blockers (ARBs) in nondialysis patients for better renal and cardiovascular outcomes has been validated by randomized clinical trials and recommended by many guidelines. Renin angiotensin system (RAS) blockade with either ARBs or ACE inhibitors was also associated with a decrease in mortality in HD and PD patients, independent of blood pressure goals.[5460] Furthermore, Moist et al.[30] reported that treatment with ACE inhibitors was independently associated with lower risk of RKF loss in PD patients (AOR = 0.69, P = 0.02). A similar outcome was also reported in a randomized trial by Suzuki et al.[61] on CAPD patients who were treated with valsartan. Zhang et al.[62] conducted a systematic review that included six studies with 257 PD patients and looked into the use of ARBs and ACE inhibitors. Prolonged use of ARBs (>12 months) was associated with better preservation of RKF in CAPD patients (MD 1.11 ml/min/1.73 m2, 95% CI: 0.38 to 1.83), while using ramipril was also associated with slower decline of RKF in CAPD (MD −0.93 mL/min/1.73 m2, 95% CI: −0.75 to −0.11) and the rate of progression to anuria. These are small RCTs with small numbers of participants however, making it difficult to avidly recommend the use of ARBs or ACE inhibitors for the preservation of RKF in PD patients. Nonetheless, we agree that using ACE inhibitors or ARBs should be encouraged in PD patients, particularly in individuals with more RKF and those with cardiovascular comorbidities.
Diuretics
Diuretics are commonly used among PD patients with RKF for blood pressure control, maintaining euvolemia and minimizing the need for higher glucose-containing solutions. The effect of diuretics on RKF is still unclear and previous studies have reported mixed results. In a well-conducted study by Medcalf et al.,[37] the use of diuretics in new CAPD patients was associated with an increase in urine volume at 1 year compared to controls. However, no difference was found in GFR. Another study that examined the use of high-dose furosemide (2 g) over 24 h in CAPD patients found it to be effective in increasing the urine volume by a median of 400 ml (range 270–910 ml, P < 0.02), but had no effect on GFR.[38] In two studies from Taiwan, the use of diuretics was associated with faster RKF decline.[3663] It is unclear if an increase in urine volume from diuretics translates into better outcome in PD patients. While maintaining adequate volume control is of paramount importance in dialysis patients, volume depletion is a major concern whenever diuretics are started and it must be avoided. It is reported that diuretics are still underutilized in dialysis patients despite their advantages.[64]
Aminoglycoside antibiotics
Aminoglycoside antibiotics are commonly used for the treatment of PD peritonitis as well as blood stream–related infection. They provide an excellent coverage for gram-negative bacteria and can be used with cephalosporin for synergy. Aminoglycosides are recommended by ISPD as an empirical antibiotic coverage for suspected PD peritonitis.[65]
Three studies that assessed the effect of aminoglycoside on RKF found no association with the rate of RKF decline.[666768] Baker et al.[67] observed no difference in loss of RKF among three groups, a group treated with aminoglycoside-based regimen (70 peritonitis episodes) versus no aminoglycoside regimen group (61 peritonitis episodes) versus 74 control patients without peritonitis. The median treatment duration was 14 days. Lui et al.[68] also did not find any difference in RKF loss in CAPD patients when peritonitis was treated with cefazolin plus netilmicin versus cefazolin plus ceftazidime. Ototoxicity remains a major concern with the use of aminoglycosides,[69] probably for longer duration and with repetitive use. Oral N-acetylcysteine therapy has been recommended by the ISPD guidelines to prevent ototoxicity, but not loss of RKF. Aminoglycoside antibiotics remain an effective antimicrobial agent and should not be withheld when indicated. It is important to note that the effect of peritonitis on RKF loss is more detrimental than the type of antibiotic used and can be irreversible. Therefore, every effort should be made for early recognition and timely antimicrobial treatment to avoid morbidity and mortality.[4367]
Iodinated contrast medium
Acute kidney injury associated with iodinated contrast exposure was reported to be as high as 15%–55% among individuals with preexisting kidney disease.[70] Moranne et al.[71] did not find a significant difference at 2 weeks following radioiodine contrast administration in PD patients with regard to RKF (measured by the average of 24-h urinary urea and creatinine clearance), daily urine volume, and peritoneal creatinine clearance. The patients were given precontrast hydration, and last-generation iodinated contrast medium was used in the study, as it is associated with significantly reduced nephrotoxicity in patients with chronic kidney disease.[72] In a retrospective study,[73] 29 patients underwent coronary angiogram, of which only one individual developed anuria and the remaining subjects showed similar rate of RKF decline to that of the control group. All patients received low osmolar iodinated contrast media, while 50% received preprocedure intravenous (IV) fluid and N-acetylcysteine. In another study,[74] there was a transient decline in RKF after an elective intra-arterial administration of contrast media. However, RKF was not different at day 30 from baseline. The PRESERVE trial failed to show any therapeutic effect of N-acetylcysteine in prevention of contrast nephropathy, although in a meta-analysis, N-acetylcysteine was associated with lower incidence of contrast-induced nephropathy and the effect was more pronounced in patients with chronic kidney disease.[7576] It is our practice to premedicate our PD patients with RKF with N-acetylcysteine before exposing them to radiocontrast agents. Figure 2 demonstrates the factors reported to affect RKF in PD patients.
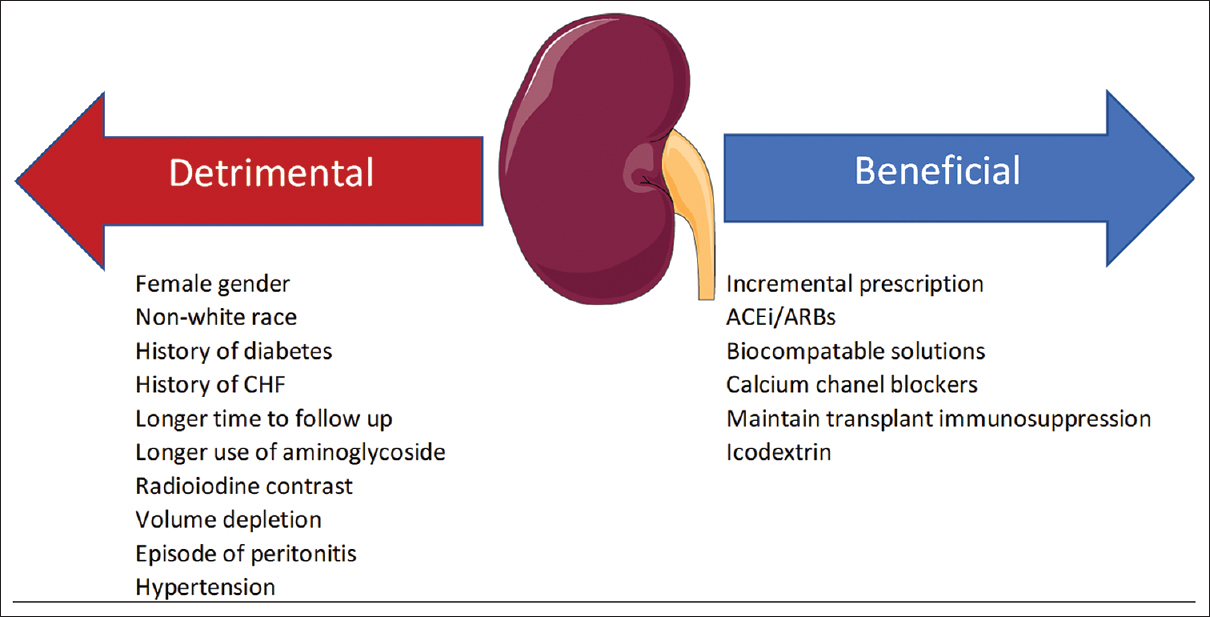
- Factors reported to affect residual kidney function. ACEi: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers. The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license
Conclusion
RKF is an important prognostic indicator in PD patients and is associated with better survival overall. Its value goes beyond just additional clearance and volume control. It is an important task for nephrologists to implement strategies that focus on RKF preservation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Adequacy of dialysis and nutrition in continuous peritoneal dialysis:Association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1996;7:198-207.
- [Google Scholar]
- Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis:A reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158-62.
- [Google Scholar]
- Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant. 1995;10:2295-305.
- [Google Scholar]
- Importance of dialysis adequacy in mortality and morbidity of Chinese CAPD patients. Kidney Int. 2000;58:400-7.
- [Google Scholar]
- The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life:An analysis of the netherlands cooperative study on the adequacy of dialysis (Necosad)-2. Am J Kidney Dis. 2003;41:1293-302.
- [Google Scholar]
- Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis. 1999;33:523-34.
- [Google Scholar]
- Predictive value of brain natriuretic peptides in patients on peritoneal dialysis:Results from the ADEMEX trial. Clin J Am Soc Nephrol. 2008;3:407-15.
- [Google Scholar]
- Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis:ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307-20.
- [Google Scholar]
- Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients:The choices for healthy outcomes in caring for end-stage renal disease (CHOICE) study. Am J Kidney Dis. 2010;56:348-58.
- [Google Scholar]
- Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transplant. 2001;16:2207-13.
- [Google Scholar]
- Independent effects of residual renal function and dialysis adequacy on nutritional status and patient outcome in continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1999;34:1056-64.
- [Google Scholar]
- Independent effects of residual renal function and dialysis adequacy on dietary micronutrient intakes in patients receiving continuous ambulatory peritoneal dialysis. Am J Clin Nutr. 2002;76:569-76.
- [Google Scholar]
- Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol. 2004;15:2186-94.
- [Google Scholar]
- A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int. 2002;62:639-47.
- [Google Scholar]
- Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant. 2007;22:2653-8.
- [Google Scholar]
- Residual renal function predicts outcome of fungal peritonitis in peritoneal dialysis patients. Perit Dial Int. 2006;26:407-9.
- [Google Scholar]
- Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int. 2005;25:274-84.
- [Google Scholar]
- Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int. 2000;20:429-38.
- [Google Scholar]
- Glomerular filtration rate:Determination in patients with chronic renal disease. JAMA. 1967;199:252-6.
- [Google Scholar]
- Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1996;7:745-50.
- [Google Scholar]
- Rationale and strategies for preserving residual kidney function in dialysis patients. Am J Nephrol. 2019;50:411-21.
- [Google Scholar]
- Estimating residual kidney function in dialysis patients without urine collection. Kidney Int. 2016;89:1099-110.
- [Google Scholar]
- Low-molecular weight proteins as markers for glomerular filtration rate. Clin Chem. 2001;47:2179-80.
- [Google Scholar]
- Utility of cystatin C-derived equations for evaluation of residual renal function in peritoneal dialysis patients. Ren Fail. 2015;37:50-6.
- [Google Scholar]
- Estimation of residual glomerular filtration rate in dialysis patients from the plasma cystatin C level. Nephrol Dial Transplant. 2007;22:1633-8.
- [Google Scholar]
- Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652-60.
- [Google Scholar]
- C-terminal agrin fragment (CAF) as a serum biomarker for residual renal function in peritoneal dialysis patients. Int Urol Nephrol. 2015;47:391-6.
- [Google Scholar]
- Low serum bicarbonate predicts residual renal function loss in peritoneal dialysis patients. Medicine (Baltimore). 2015;94:e1276.
- [Google Scholar]
- Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556-64.
- [Google Scholar]
- Peritoneal dialysis or hemodialysis?A dilemma for the nephrologist. Adv Perit Dial. 2006;22:180-5.
- [Google Scholar]
- Peritoneal dialysis retardation of progression of advanced renal failure. Perit Dial Int. 2002;22:239-42.
- [Google Scholar]
- Rate of decline in residual kidney function pre and post peritoneal dialysis initiation:A post hoc analysis of the IDEAL study. PLoS One. 2020;15:e0242254.
- [Google Scholar]
- Rate of decline of residual kidney function before and after the start of peritoneal dialysis. Perit Dial Int. 2016;36:334-9.
- [Google Scholar]
- Comparative outcomes between continuous ambulatory and automated peritoneal dialysis:A narrative review. Am J Kidney Dis. 2014;63:1027-37.
- [Google Scholar]
- Predictors of faster decline of residual renal function in Taiwanese peritoneal dialysis patients. Perit Dial Int. 2008;28(Suppl 3):S191-5.
- [Google Scholar]
- Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001;59:1128-33.
- [Google Scholar]
- Acute effects of high-dose furosemide on residual renal function in CAPD patients. Perit Dial Int. 2003;23:339-47.
- [Google Scholar]
- Effect of Kt/V on survival and clinical outcome in CAPD patients in a randomized prospective study. Kidney Int. 2003;64:649-56.
- [Google Scholar]
- Incremental peritoneal dialysis-definition, prescription, and clinical outcomes. Kidney360. 2023;4:272-7.
- [Google Scholar]
- The importance of residual kidney function for patients on dialysis:A critical review. Am J Kidney Dis. 2009;53:1068-81.
- [Google Scholar]
- single daily icodextrin exchange as initial and solitary therapy. Perit Dial Int. 2018;38:119-24.
- [Google Scholar]
- Incremental peritoneal dialysis allows to reduce the time spent for dialysis, glucose exposure, economic cost, plastic waste and water consumption. J Nephrol. 2023;36:263-73.
- [Google Scholar]
- Three versus 4 daily exchanges and residual kidney function decline in incident CAPD patients:A randomized controlled trial. Am J Kidney Dis. 2017;69:506-13.
- [Google Scholar]
- The role of incremental peritoneal dialysis among patients on peritoneal dialysis. A longitudinal analysis over 20 years. Port J Nephrol Hypert. 2021;35:22-8.
- [Google Scholar]
- Incremental peritoneal dialysis:New ideas about an old approach. Semin Dial. 2018;31:445-8.
- [Google Scholar]
- Ico-Alone“single nocturnal exchange to initiate peritoneal dialysis in patients with residual renal function-Five year, single centre experience. Indian J Nephrol. 2013;23:276-9.
- [Google Scholar]
- Incremental peritoneal dialysis in incident end-stage kidney disease patients. Perit Dial Int. 2022;42:387-93.
- [Google Scholar]
- International Society for Peritoneal Dialysis practice recommendations:Prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int. 2020;40:244-53.
- [Google Scholar]
- Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol. 2012;23:1097-107.
- [Google Scholar]
- Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev 2014:Cd007554.
- [Google Scholar]
- Effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in dialysis patients:A nationwide data survey and propensity analysis. Medicine (Baltimore). 2015;94:e424.
- [Google Scholar]
- Slouching towards Bethlehem:The beast of biocompatibility. Nephrol Dial Transplant. 2010;25:2050-1.
- [Google Scholar]
- Echocardiographic, electrocardiographic and blood pressure changes induced by icodextrin solution in diabetic patients on peritoneal dialysis. Kidney Int Suppl. 2008;108:S125-30.
- [Google Scholar]
- Effect of icodextrin solution on the preservation of residual renal function in peritoneal dialysis patients:A randomized controlled study. Medicine (Baltimore). 2016;95:e2991.
- [Google Scholar]
- Comparison of icodextrin and glucose solutions for long dwell exchange in peritoneal dialysis:A meta-analysis of randomized controlled trials. Perit Dial Int. 2011;31:179-88.
- [Google Scholar]
- Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev. 2018;10:Cd007554.
- [Google Scholar]
- Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use and cardiovascular outcomes in patients initiating peritoneal dialysis. Nephrol Dial Transplant. 2017;32:862-9.
- [Google Scholar]
- Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis. 2004;4:1056-64.
- [Google Scholar]
- Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for preserving residual kidney function in peritoneal dialysis patients. Cochrane Database Syst Rev. 2014;2014:CD009120.
- [Google Scholar]
- Predictors of residual renal function decline in patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int. 2015;35:180-8.
- [Google Scholar]
- ISPD peritonitis guideline recommendations:2022 update on prevention and treatment. Perit Dial Int. 2022;42:110-53.
- [Google Scholar]
- Use of aminoglycosides for peritoneal dialysis-associated peritonitis does not affect residual renal function. Nephrol Dial Transplant. 2012;27:381-7.
- [Google Scholar]
- Empirical aminoglycosides for peritonitis do not affect residual renal function. Am J Kidney Dis. 2003;41:670-5.
- [Google Scholar]
- Cefazolin plus netilmicin versus cefazolin plus ceftazidime for treating CAPD peritonitis:Effect on residual renal function. Kidney Int. 2005;68:2375-80.
- [Google Scholar]
- Correlation between hearing loss and peritonitis frequency and administration of ototoxic intraperitoneal antibiotics in patients with CAPD. Ren Fail. 2010;32:179-84.
- [Google Scholar]
- Contrast-induced nephropathy:Definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006;69:S11-5.
- [Google Scholar]
- Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant. 2006;21:1040-5.
- [Google Scholar]
- Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348:491-9.
- [Google Scholar]
- The effect of coronary angiography on residual renal function in patients on peritoneal dialysis. Clin Cardiol. 2006;29:494-7.
- [Google Scholar]
- Effect of radio contrast media on residual renal function in peritoneal dialysis patients--a prospective study. Nephrol Dial Transplant. 2006;21:1334-1339.
- [Google Scholar]
- Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med. 2018;378:603-14.
- [Google Scholar]
- The effectiveness of N-acetylcysteine in preventing contrast-induced nephropathy in patients undergoing contrast-enhanced computed tomography:A meta-analysis of randomized controlled trials. Int Urol Nephrol. 2013;45:1309-18.
- [Google Scholar]







