Translate this page into:
ABO-Incompatible Renal Transplant: A Single-Center Experience from India
Corresponding Author: Dr. Vaibhav Tiwari, Department of Nephrology, Sir Ganga Ram Hospital, Old Rajinder Nagar, New Delhi - 110 060, India. E-mail: drvt87@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pawar N, Tiwari V, Gupta A, Divyaveer S, Rather I, Chadha S, et al. ABO-Incompatible Renal Transplant: A Single-Center Experience from India. Indian J Nephrol 2024;34:24-30. doi: 10.4103/ijn.ijn_247_22
Abstract
Introduction:
In view of ever-increasing end-stage renal disease (ESRD) population but inadequate availability of suitable donors, ABO-incompatible (ABOi) transplantation can be an important void filler. However, at present, ABOi transplantation is limited to a few centers in India and there is a lack of adequate experience and expertise to guide this program to other centers in the country.
Methods:
Data of all the ABOi transplants performed from 2012 to 2021 in a tertiary care hospital was retrospectively analyzed. The anti-ABO antibody (IgG) titers (≤1:4) were considered safe before transplantation. Desensitization included rituximab, plasma exchange, or selective immunoadsorption column. Tacrolimus and mycophenolate mofetil were initiated at day −7. Induction agents included ATG, ATLG, basiliximab, or no induction. Postoperatively, anti-ABO titers were done daily for 2 weeks.
Results:
A total of 202 patients underwent transplantation; of these, 195 patients whose data were for available for 12 months were included in the study. Mean duration of follow-up was 28.9 ± 21.7 months. UTI was the most common source of infection, occurring in almost half (46.1%) of the patients. Antibody-mediated rejection (ABMR; 15%) was common in the first year. Patient survival was 86.6% (169/195) at 1 year. Sepsis was the most common of death in more than two-thirds of the population, including coronavirus disease 2019 (COVID-19)–associated mortality in nine patients (4.6%). Death-censored graft survival was 89.3% (174/195). AMR was the leading cause of graft loss in almost half of the patients.
Conclusion:
ABOi should be considered in ESRD patients for whom suitable ABO-compatible donor is not available. Higher rate of rejection and infection are still a major concern.
Keywords
ABO incompatible
infection
rejection
transplant
Introduction
Chronic kidney disease is a major contributor to morbidity and mortality from noncommunicable diseases.1 The program of living kidney transplantation has evolved in India in the last 40 years. Currently, in India, around 220,000 individuals require kidney replacement therapy annually, but it mostly remains unmatched as 7500 kidney transplants are being performed at approximately 250 centers across the country.2 The majority (>90%) are from the living donor program and the remaining 10% are from the deceased donor program.3
For a long time, ABO incompatibility was a contraindication for a kidney transplant. Initial period of ABO-incompatible (ABOi) living kidney transplantation had a very high rejection rate;4,5 histopathology showed arterial thrombosis and parenchymal necrosis.
This changed in 1982 when Alexander et al. published a large study on ABOi kidney transplantation with successful desensitization with plasmapheresis, splenectomy, donor thymocyte transfusion together with intense immunosuppression. Graft survival at 1 year was 75%.6 Later, the Swedish team of Tydén et al. began using rituximab, a chimeric anti-CD20 antibody instead of splenectomy, with excellent short-term outcomes for ABOi living kidney transplant.7 Johns Hopkins and Mayo Clinic teams reported excellent outcomes for ABOi kidney transplantation using various induction protocols that included rituximab.8 Japan has also been a major contributor to ABOi kidney transplantation since the late 1980s. A 2006 Japanese registry analysis showed that ABOi kidney transplant survival rates were acceptable but still lower than those of ABO-compatible (ABOc) transplant. Later, a follow-up analysis of Japanese recipients from 2001 to 2010 showed better outcome with the use of rituximab, mycophenolate mofetil, and tacrolimus.9 Over the last 20 years, with a better understanding of the mechanism of rejection and improvement in the technique of removal of specific isoagglutinin, eligibility for living donor programs has been extended. In a developing country like India, it is still in its infancy. Anecdotal experience suggests that approximately 200–250 ABOi kidney transplants are performed annually in India.2
Herein, we report our experience of ABOi kidney transplantation over 9 years (2012–2021).
Materials and Methods
We retrospectively analyzed all the patients who underwent ABOi transplantation at Sir Gangaram Hospital, New Delhi from 2012 to 2021.
Pretransplantation protocol
All patients were subjected to tier 1 crossmatch including complement-dependent cytotoxicity crossmatch, panel-reactive antibody, and donor-specific antibody crossmatch by lysate method on the Luminex platform. Any patient who had positive or borderline results in any of the tier 1 crossmatches was subjected to flow crossmatch. Along with that, any patient who was considered high risk (e.g., second transplant, recent blood transfusion, husband to wife donation) was also subjected to flow crossmatch. Single antigen bead was performed in whom flow crossmatch was positive or borderline. From 2018 onward, all the high-risk patients, and the patients who had positive or borderline tier 1 crossmatch, were also subjected to the single antigen bead.
Antibody titer determination
Anti-ABO titer was measured by the column agglutination test. using DiaMed ID Micro Typing system (Bio-Rad, Hercules, CA, USA) or BioVue System (Ortho Clinical Diagnosis, Raritan, NJ, USA) according to the availability of the kit. In these assays, plasma from the patient is stepwise diluted 1:2 with normal saline and packed red blood cells (RBCs) are used to make a suspension with a cell stabilization solution. After incubation and centrifugation, agglutination is observed in the card or cassette. Negative test cells settle at the bottom of the column and positive cells are captured at the top of or within the body of the column. The gel traps the RBC agglutinates as a filter during centrifugation. The agglutination is graded from 0 to 4+.
Desensitization protocol
Preconditioning protocol [Figure 1] consisted of removal of isoagglutinins and rituximab. The protocol evolved over a period of time with our experience, and modifications based on the increasing volume data on ABOi Kidney Transplantation became available. A titer of IgG≤1:4 was considered safe before the transplant. Reduction of B-lymphocyte pool by using anti-CD20, rituximab 500 or 200 mg, as a single dose, given 14 days before transplant. Patients before 2018 received 500 mg of rituximab, while patients after 2018 received 200 mg of rituximab. Anti-A/B antibody depletion at the time of transplantation by doing plasma exchanges on an alternate day was done with 100% volume replacement with 20% albumin and crystalloids. This process reduces anti-ABO antibodies by 20% in each session.10 Another method was using selective A/B immunoadsorption (IA) columns Glycosorb or Adsopak. IA columns are reused after saline wash and sterilization with ethylene oxide with storage in dark at 2°C–8°C for three or four times.2 Study done at our center on the efficacy of reuse of IA columns showed the reduction of anti-ABO titer was 4 logs after the first use, 3 logs after the first reuse, and 1.5 logs after the second reuse.11 Tacrolimus (0.05 mg/kg/day) and mycophenolate mofetil (1 g/day) were started 1 week before transplant. Based on the recipient’s immunological risk profile, either no induction was given or anti-thymoglobulin, anti-T-lymphocyte globulin, or basiliximab was used. Anti-thymoglobulin (thymoglobulin by Sanofi Genzyme) was given as 1.5 mg/kg/dose. A total of two or three doses were given according to risk factors. Anti-T-lymphocyte globulin (Grafalon by Zydus Cadila) was given as a total dose of 5–10 mg/kg over 3–4 days. Basiliximab (Simulect by Novartis) was given in two divided doses –20 mg on 0 day and the fourth day. All patients received full-dose immunosuppression from day 2. Tacrolimus was given at 0.1 mg/kg/day in two divided doses to maintain a trough level of 8–10 ng/mL for 0–3 months, 6–8 ng/mL for 3–6 months, and 4–6 ng/mL thereafter. Tacrolimus levels were measured by chemiluminescent microparticle immunoassay (CMIA). Cyclosporine levels, that is, C0, was maintained at 200–300 ng/mL in months 1–3 and 50–150 ng/mL for subsequent months and C2 was maintained at 800–1000 ng/mL in the first 3 months and 400–600 ng/mL for subsequent months. Mycophenolate mofetil was given at 1.5–2 g in two to three divided doses. Methylprednisolone 500 g on the day of transplant, then 250 mg till day 3. It is then shifted to oral steroid, prednisolone 0.5 mg/kg/day and tapered to 7.5–5 mg/day at 3 months and continued thereafter at the same dose as per the center’s protocol.

- Desensitization and immunosuppressive protocol for ABO-incompatible transplant.
Postoperative ABO titers
ABO antibody (IgG and IgM) titers were measured daily at 8 AM. If the rise of titer persisted or a rise in serum creatinine (>0.3 mg/dL) in 48 h occurred with titers‚ ≥1:16, a kidney biopsy was planned. After 2 weeks, the anti-ABO titer was done weekly or whenever there was an unexplained rise in creatinine till 6 weeks.
Prophylaxis
Cotrimoxazole was given for 6 months and valganciclovir for 3 months as prophylaxis in all patients with immediate graft function.
Follow-up
All patients were followed up thrice a week for 2 weeks, then twice a week till the second month, weekly in the third month, twice a month in 4–6 months, monthly till 1 year, then every two to three monthly.
Data collection
Baseline characteristics were assessed from clinical records. Basic diagnoses of chronic interstitial nephritis and chronic glomerulonephritis were inferred on the basis of clinical and biochemical data, although it could not be ascertained in all cases. Follow-up data were collected from the medical record section and the outpatient department (OPD) facilities. Graft function was assessed by serial measurement of serum creatinine during each visit. Data was collected till June 2021, at which time graft survival and patient survival were assessed. Infectious complications recorded comprised Urinary Tract Infection (UTI) bacterial infections, tuberculosis, cytomegalovirus (CMV), BK virus (BKV), pneumocystis pneumonia and fungal infections.
We treated biopsy-proven ABMR by plasmapheresis, 40 mL/kg, followed by Intravenous immunoglobulin (IVIG) at a dose of 100–200 mg/kg after each session. Each patient also received methylprednisolone 250–500 mg/dose, two to three doses, according to the response and the risk of infection. The volume replacement was done with albumin and fresh frozen plasma. Other immunosuppressive drugs were modified according to the trough levels.
We treated biopsy-proven ACR with augmentation of immunosuppression and pulse steroid 500 mg for 1–3 days according to the response. Antithymocyte globulin (ATG) (1–1.5 mg/kg/dose) was given to nonresponding patients.
Borderline rejection was treated with augmentation of immunosuppression and pulse steroid according to the response. Augmentation of immunosuppression included maintaining the Calcineurin inhibitors (CNIs) level in the upper range according to the period after transplant, increment in the dose of antiproliferative agent, and continuing steroids in slightly higher doses in maintenance (e.g., 7.5 mg/day instead of 5 mg/day).
Statistical analysis
Continuous variables are reported as mean ± standard deviation, and categorical variables are reported as percentages. Kaplan–Meier survival analysis was performed for graft and patient survival rates. The statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software version 23.0 (IBM, Chicago, IL, USA).
Results
A total of 202 ABOi renal transplants were done from June 2012 to June 2021. Patients with at least 6 months of follow-up were included. Complete data of at least 1 year were available for 195 patients, and only these were included in the final analysis. Mean duration of follow-up was 28.9 ± 21.7 months. Baseline characteristics of the recipients and donors are presented in Table 1.
| Variables | Value |
|---|---|
| Number, n | 195 |
| Recipient | |
| Age, mean±SD | 40.9±12 |
| Sex (M, %) | 83.50% |
| Basic disease | |
| Diabetic kidney disease | 52 (27%) |
| Chronic glomerulonephritis | 72 (37%) |
| Chronic interstitial nephritis | 24 (12%) |
| Others | 47 (24%) |
| Comorbidity | |
| Coronary artery disease | 28 (14%) |
| Cerebrovascular accident | 10 (5%) |
| Peripheral vascular disease | 3 (1.5%) |
| Malignancy | 0 |
| Donor | |
| Age, mean±SD (years) | 48.7±7.2 |
| Sex (M, %) | 38 (19%) |
| Relationship | |
| Wife | 95 (48%) |
| Mother | 36 (18%) |
| Sister | 9 (5%) |
| Husband | 9 (5%) |
| Others | 46 (24%) |
| Antibody titer at baseline | |
| High (>1:128) | 34 (17.4%) |
| Low (<1:128) | 161 (83%) |
| HLA mismatch | 4±2.1 |
| Preemptive transplantation | 14 (7%) |
HLA=human leukocyte antigen, SD=standard deviation
Initial IgG agglutinin titers ranged from 1:2 to 1:1024. In the initial period of the transplantation program, single plasma exchange was done in patients with an IgG titer of ≤1:4 before transplant. One hundred and eighty-seven patients received plasma exchange. The average number of plasma exchanges done was 3.3 (range 1–10). Of these, 45 patients (23%) were also desensitized by the IA column (Glycosorb or Adsopak).The IA column option was given to patients with a baseline titer >1:32.
Induction was given to 188 patients, while no induction was given to seven (3.4%) patients. ATG was used in 122 (62.5%), followed by Anti T-lymphocyte Immunoglobulin (ATLG) in 48 (24.6%) and basiliximab in 18 (9.2%) cases.
Hyperacute rejection was not seen in any recipient. A total of 71 graft biopsies were done in the first year in 62 patients [Table 2]. Antibody mediated rejection (AMR) was the most common diagnosis (41.4%).
| Infection | n (%) |
|---|---|
| Urinary tract infection | 90 (46%) |
| Lower respiratory tract infection | 25 (13%) |
| TB | 3 (1.6%) |
| COVID-19 | 14 (7.2%) |
| Opportunistic infection | |
| Aspergillus | 3 (1.6%) |
| CMV | |
| Mucormycosis | 3 (1.6%) |
| Pneumocystis carinii | 5 (2.6%) |
| Nocardiosis | 2 (1.0%) |
| Cryptococcal meningitis | 2 (1.0%) |
CMV=cytomegalovirus, COVID-19=coronavirus disease 2019, TB=tuberculosis
Infections in the first year of transplant are shown in Table 3. Urinary tract infections were the most common, present in 90 (46.1%) cases, with Escherichia coli (67%), Klebsiella pneumoniae (22%), and Pseudomonas aeruginosa (5%) being the most common pathogens. In symptomatic patients who were screened for CMV and BKV infections, CMV viremia was positive in 16 (8.2%) cases, of which 14 patients received ATG as induction and the other two received basiliximab. All CMV infections occurred after CMV prophylaxis, while BKV infection was present in two cases. Lower respiratory tract infection was found in 25 (12.8%) cases- Aspergillus three (1.5%), Mucor three (1.5%), tuberculosis three (1.5%), Pneumocystis carinii three (1.5%), and the rest were bacterial pneumonia. During the coronavirus disease 2019 (COVID-19) wave, 14 patients were detected with COVID-19 and of them, nine (64.2%) patients succumbed to death. One patient had acute coronary syndrome within 1 month of transplant and underwent cardiac stenting.
| Biopsy | n (%) |
|---|---|
| Antibody-mediated rejection | 29 (14.8%) |
| ACR | 3 (1.5%) |
| Borderline ACR | 9 (4.6%) |
| ATN with no rejection | 15 (7.6%) |
| Acute CNI toxicity | 8 (4.1%) |
| BK virus nephropathy | 2 (1%) |
| Normal | 5 (2.5%) |
| Total | 71 (36.4%) |
ACR=acute cellular rejection
Patient survival at 1 year was 169 (86.6%) patients [Figure 2a]. The most common cause of death included sepsis in 73% (19/26) and cardiovascular events in 11.5% (3/26) of patients.
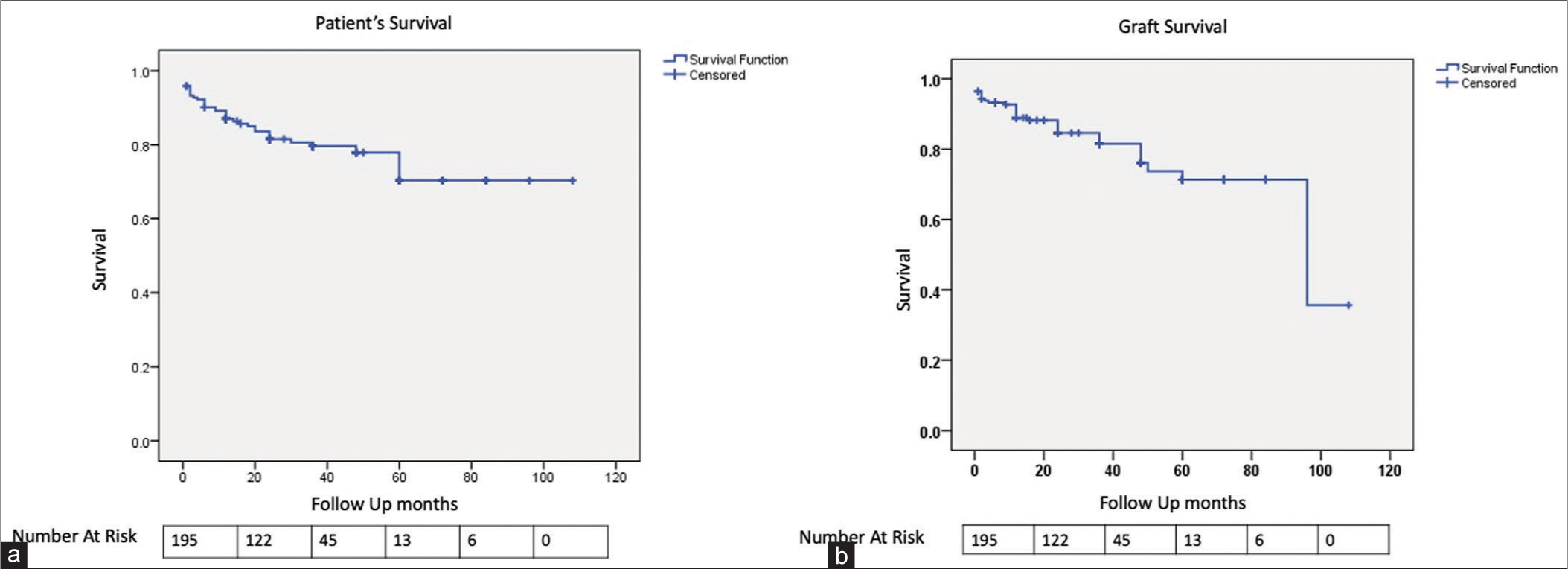
- Kaplan–Meier graph for (a) patient survival and (b) graft survival.
Death-censored graft survival was seen in 174 (89.3%) patients within 1 year [Figure 2b]. Mean creatinine at the end of the first year was 1.44 ± 0.51 mg/dL. AMR was the most common finding, seen in 57% (12/21) patients, of which acute cortical necrosis was seen in three cases. Acute CNI toxicity was seen in 19% (4/21) and sepsis was associated with ATN in 19% (4/21) patients.
Graft nephrectomy was done in three patients due to graft loss within 3 months of the transplant. But none of them was done in the first 2 weeks of the transplant.
Discussion
This paper describes the largest single centre experience of ABOi transplants in India. Though expanding rapidly, ABOi has been limited to a few centers due to a lack of resources, experience, and technical expertise. Till the preparation of this manuscript, data from only 400 patients have been published in literature from India [Table 4].12-20
| Center | MOIT Hospital, Chennai[12] |
Medanta, Gurgaon[13] |
RTICS, Kolkata[14] |
RTICS, Kolkata[15] |
Fortis, Medanta, Gurgaon[16] |
R&R, Delhi[17] |
Medanta, Gurgaon[18] |
Medanta, Gurgaon[19] |
MJUH, Nadiad[20] |
Present study |
|---|---|---|---|---|---|---|---|---|---|---|
| Year of the study | 2010–2014 | 2011–2014 | 2013–2015 | 2014–2015 | 2011–2018 | 2014–2018 | 2017–2018 | 2011–2020 | 2013–2019 | 2012–2021 |
| Follow-up (months) | 37.6 | 10 | 12 | 12 | 31 | 30 | 11 | 33 | 26 | 28 |
| No. of patients | 18 | 20 | 45 | 30 | 50 | 30 | 5 | 100 | 100 | 195 |
| Death-censored graft survival (%) | 100 | 95 | 97.80 | 96.7 | 88 | 100 | 100 | 93 | 73.50 | 89.3 |
| Patient survival (%) | 100 | 90 | 97.80 | 96.7 | 94 | 86.7 | 100 | 94 | 93.30 | 87.10 |
| UTI (%) | 22 | - | - | 3 | - | - | - | 18 | 49 | 46 |
| Rejection (%) | 0 | 15 | 2.22 | 3 | 22 | 10 | 0 | 17 | 28 | 16 |
| ABMR (%) | 0 | 0 | 2.22 | 0 | 8 | 3 | 0 | 3 | 15 | 15 |
| ACR (%) | 0 | 15 | 0 | 0 | 14 | 0 | 0 | 14 | 10 | 2 |
| Mixed (%) | 0 | 0 | 0 | - | 0 | 7 | 0 | - | - | 0 |
| BCR (%) | - | 5 | - | - | - | - | - | - | - | 4.6 |
ABMR=antibody-mediated rejection, ACR=acute cellular rejection, BCR=borderline cellular rejection, UTI=urinary tract infection
Over the period of 9 years, various modifications were incorporated into the course. Firstly, the dose of rituximab was modified. In 2018, the dose was reduced from 500 to 200 mg. This was primarily done in view of increasing global data suggesting lower doses to be equally effective.21,22 With the introduction of selective anti-A/B columns in 2018, more patients with higher antibody titers (IgG >1:128) were included in the study. However, initial titers were not able to predict successful reduction as seen in a previous study from the same institution.11 Two to three log reduction in a single session with columns was achieved, but a similar or higher rebound was also observed. To tackle this, we introduced a novel technique to reuse the columns while maintaining efficacy and safety.11
Due to desensitization and immunosuppression, infection is a major concern among recipients. In our study, 146 episodes of infection were reported among 125 patients in the first year. UTI was the most common infection among 46% of the recipients. This is slightly higher than a study from our institute which reported a rate of 35.6% among all renal transplant patients.23 A similar trend has been seen in other studies from India, with the rate of infection ranging from 25% to 63% and with UTI being the most common cause of sepsis.12,18,20,24
During the first year, biopsy-proven acute rejection (BPAR) was found in 16% of the patients, with majority having AMR, including 4% of patients having Thrombotic Microangiopathy (TMA) and chronic allograft nephropathy/interstitial fibrosis and tubular atrophy resulting in graft loss. None of the patients had raised anti-ABO antibodies at the time of rejection. Before transplantation, none of the patients had positive Donor specific Antibody (DSA). DSA was not repeated at the time of rejection in all cases. The rate of AMR in ABOi compared to ABOc transplant has been reported to be significantly higher in various meta-analyses.19,25 In the meta-analysis by de Weerd et al. that analyzed 1346 ABOi patients, significantly higher relative risk of AMR of 3.86 (95% confidence interval [CI], 2.05–7.29, p = 0.001) was reported compared to ABOc.19 Similarly, a higher rate of AMR was found by Scurt et al. in a meta-analysis of 7098 ABOi patients.25 As there was no case of AMR due to anti-ABO antibodies, we were unable to differentiate the phenotypic difference between anti-human leukocyte antigen (anti-HLA) and anti-ABO ABMR. Graft survival has been variable among other Indian studies, with rates ranging from 100% to 70% [Table 4]. Higher AMR generally translates into poor graft survival. Unsurprisingly, death-censored graft survival in this report was 89.3%, with AMR being the most common cause of graft loss. The immunological disadvantage with older age, higher human leukocyte antigen (HLA) mismatches, unrelated donors, higher Panel Reactive Antibody (PRA) and DSAs, and ineffectiveness of rituximab in preventing de novo DSA, all result in higher chances of AMR and poorer graft survival among ABOi.19
The role of baseline anti-ABO antibody titers in ABMR and graft survival is a matter of debate. Various studies have evaluated the outcome with reference to baseline titers; however, most of them differ in the dose of rituximab, IVIg, and the requirement of splenectomy.26-31 In the study by Won et al., despite having higher ABO titers in the post-op period, there was no difference in graft survival.31 In a comparative study by Chung et al.,27 the outcomes of high (>1:256) and low (<1:256) baseline antibody titer groups were similar when the ABO titers were kept below <1:32. Several other studies have suggested a negative impact of baseline titers on graft survival.27 In the present study, we did not find any difference between the two groups (>1:256 vs. <1:256) in terms of patient and graft survival. Similarly, we did not find a rebound of titers in the high-titer group. One of the reasons for the low rebound could be the achievement of very low titers (<1:4) at the transplant, which was much lower than in all other studies. In the study by Chung et al., the authors have suggested achieving and maintaining the titer as <1:32 to prevent AMR.27
Patient survival was reported at 86.6%. Urosepsis and COVID-19 were the most common causes of death. Antibody removal and rituximab are the most important factors among others which differentiate the journey of an ABOi and ABOc before transplantation and make the patient much more immunosuppressed than ABOc. In the present study, the dose of rituximab was decreased to 200 mg from 500 mg; however, no significant reduction in infection was observed. Subanalysis of selective IA columns versus Plasma Exchange (PLEX) did not show any significant difference in this study, although the number of the IA columns in patients was small. A study comparing IA with PLEX to assess the impact infection rate is needed. In the meta-analysis by de Weerd et al., the authors found poorer survival outcomes in ABOi (98% vs. 99%, P = 0.03).19 The relative risk for 1-year patient survival in ABOi patients was 0.99. Similar to the present study, infection was the most common cause of mortality and morbidity in India as well as in other countries, suggesting that we still need to explore further a more balanced approach for immunosuppression. One-year patient survival varied from 86% to 100% in different studies reported from India. Our patient survival is comparable to the studies reported from India. Recent pandemic of COVID has impacted greatly on patient survival. Apart from that, infections and sepsis are the important causes of poor patient survival.
There are many barriers in terms of logistical and economic issues in ABOi. In a developing country like India where the gross domestic product (GDP) per capita income is less than 8000 $ PPP (Purchasing power parity), spending an amount for surgery (10,000–15,000$) is an exorbitant event along with a lifelong expenditure of 3000–4000$ per annum.32 Another issue is the irregular follow-up due to the distance between the patient’s residence and the hospital, resulting in subsequent noncompliance and delay in treatment. Despite all these problems, in our country, survival and adherence to dialysis are very poor in hemodialysis. And, since there is an absence of national-level registries and a stagnant state run deceased donor kidney transplant program, ABOi can be a suitable alternative to both of these other modalities of renal replacement therapy.
This is the largest study from India to date reporting the outcomes of ABOi patients. However, the study’s retrospective nature, short follow-ups, and unavailability of a control arm as in the form of ABOc are some limitations of the study.
Conclusion
Higher infection and rejection rates and poor patient and graft survival are still major roadblocks to accepting ABOi as an appropriate void filler for end-stage renal disease (ESRD) patients. Further research is needed to optimise immunosuppression and to find the role of selective IA columns and the appropriate dose of rituximab, which can offer the patients and clinicians a high level of confidence.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Global, regional, and national burden of chronic kidney disease 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709-33.
- [Google Scholar]
- Knowledge, attitude, and practices regarding organ donation among adult visitors in a public hospital in Delhi, India. Indian J Transplant. 2017;11:127-32.
- [CrossRef] [Google Scholar]
- Current trends in kidney transplantation in India. Indian J Urol. 2016;32:173-4.
- [CrossRef] [PubMed] [Google Scholar]
- Morphological aspects of renal homograft rejection. Br Med Bull. 1965;21:171-5.
- [CrossRef] [PubMed] [Google Scholar]
- ABO blood group incompatibility in human renal homotransplantation. Am J Clin Pathol. 1969;51:15-23.
- [CrossRef] [PubMed] [Google Scholar]
- ABO-incompatible kidney transplantation. Front Immunol. 2017;8:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003;76:730-1.
- [CrossRef] [PubMed] [Google Scholar]
- Current protocols and outcomes of ABO-incompatible kidney transplantation based on a single-center experience. Transl Androl Urol. 2019;8:126-33.
- [CrossRef] [PubMed] [Google Scholar]
- Latest insights on ABO-incompatible living-donor renal transplantation. Int J Urol. 2020;27:30-8.
- [CrossRef] [PubMed] [Google Scholar]
- ABO Incompatible kidney transplantation-Current status and uncertainties. J Transplant. 2011;2011:970421.
- [CrossRef] [PubMed] [Google Scholar]
- Long term follow-up of ABO incompatible kidney transplantation-A study from India. Open Access Lib J. 2016;3:1-5.
- [CrossRef] [Google Scholar]
- ABO-incompatible renal transplantation in developing world-crossing the immunological (and mental) barrier. Indian J Nephrol. 2016;26:113-8.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of ABO-incompatible living donor renal transplantations: A single-center experience from Eastern India. Transplant Proc. 2016;48:2622-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative analysis of ABO-incompatible kidney transplantation with ABO-compatible transplantation: A single-center experience from Eastern India. Saudi J Kidney Dis Transpl. 2019;30:97-107.
- [CrossRef] [PubMed] [Google Scholar]
- ABO-incompatible renal transplantation: The journey so far on a road less traveled. Indian J Transplant. 2018;12:177-81.
- [CrossRef] [Google Scholar]
- Use of immunoadsorption columns in ABO-incompatible renal transplantation: A prospective study at a tertiary care center in India. Med J Armed Forces India. 2021;77:15-21.
- [CrossRef] [PubMed] [Google Scholar]
- ABO-incompatible kidney transplantation in India: A single-center experience of first hundred cases. Indian J Nephrol. 2022;32:42-6.
- [CrossRef] [PubMed] [Google Scholar]
- ABO-incompatible kidney transplant outcomes: A meta-analysis. Clin J Am Soc Nephrol. 2018;13:1234-43.
- [CrossRef] [PubMed] [Google Scholar]
- Kidney transplantation with ABO-incompatible donors: A comparison with matched ABO compatible donor transplants. Indian J Nephrol. 2021;31:358-64.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of rituximab dose on induction therapy in ABO-incompatible living kidney transplantation: A network meta-analysis. Medicine. 2021;100:e24853.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of rituximab dosage for ABO-incompatible living-donor kidney transplantation. Transplant Proc. 2015;47:644-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile and predisposing factors for the development of urinary tract infection during the first 3 months postrenal transplantation: A tertiary care hospital experience. Indian J Transplant. 2020;14:104-10.
- [CrossRef] [Google Scholar]
- Immunoadsorption in ABO-incompatible kidney transplantation in adult and pediatric patients with follow-up on graft and patient survival: First series from India. Asian J Transfus Sci. 2020;14:13-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcomes after ABO-incompatible renal transplantation: A systematic review and meta-analysis. Lancet. 2019;393:2059-72.
- [CrossRef] [PubMed] [Google Scholar]
- ABO-incompatible kidney transplantation. Transplantation. 2004;78:635-40.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of clinical outcome between high and low baseline anti-ABO antibody titers in ABO-incompatible kidney transplantation. Ren Fail. 2011;33:150-8.
- [CrossRef] [PubMed] [Google Scholar]
- ABO-incompatible kidney transplantation using both A2 and non-A2 living donors. Transplantation. 2003;75:971-7.
- [CrossRef] [PubMed] [Google Scholar]
- Role of anti-A/B antibody titers in results of ABO-incompatible kidney transplantation. Transplantation. 2000;70:1331-5.
- [CrossRef] [PubMed] [Google Scholar]
- Short-term outcomes of ABO-incompatible living donor kidney transplantation with uniform protocol: Significance of baseline anti-ABO titer. Transplant Proc. 2016;48:820-6.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of isoagglutinin titer in ABO-incompatible kidney transplantation. J Clin Apher. 2014;29:243-50.
- [CrossRef] [PubMed] [Google Scholar]







