Translate this page into:
Acquired Pure Red Cell Aplasia Following Recombinant Erythropoietin (Darbepoetin-alfa) Therapy
Address for correspondence: Dr. Somanath Padhi, Department of Pathology and Laboratory Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. E-mail: pathol_somanath@aiimsbhubaneswar.edu.in
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acquired pure red cell aplasia (PRCA) following use of recombinant erythropoietin (rEPO) is distinctly rare and sporadically reported in the literature. We discuss a case of PRCA following the usage of rEPO (darbepoetin-α) during the management of anemia of chronic kidney disease in an elderly male subject with review of literature and a brief insight into proposed pathophysiologic mechanism, diagnosis, and management.
Keywords
Anti-EPO antibody
erythropoiesis
erythropoietic stimulating agents
Introduction
Pure red cell aplasia (PRCA) is an uncommon hematological disorder characterized by severe anemia and reticulocytopenia (<1%) resulting due to near-total absence of maturing erythroid precursors in a morphologically normal bone marrow as a result of maturation arrest at the stage of early erythroid progenitors.[1] From an etiological point of view, acquired PRCA is reported to be associated with infections, autoimmune disorders, pregnancy, drugs such as antiepileptics, antibiotics, isoniazid, antiplatelet drugs (clopidogrel), and both solid (thymomas; up to 15% of all cases) and hematolymphoid malignancies.[2] Although the usage of recombinant erythropoietin (rEPO) in India dates back to many decades, mostly in the management of anemia of chronic kidney disease (CKD), rEPO-associated PRCA cases seem to be underreported due to lack of awareness and availability of diagnostic tests.[34567]
In this article, we aim to highlight a case of PRCA secondary to rEPO (darbopoetin-α) in an elderly male with a brief comprehensive review of literature highlighting possible pathophysiologic mechanisms, diagnostic strategies, and its management.
Case Presentation
A 76-year male patient was subjected to bone marrow aspiration (BMA), trephine biopsy (BMBx), and cytogenetics study for the evaluation of transfusion-dependent worsening anemia since the past 6 months, to rule out the possibility of myelodysplastic syndrome. He was a known case of CKD secondary to long-standing type 2 diabetic and hypertensive nephropathy and associated bilateral hydronephrosis secondary to benign prostatic hyperplasia. He had one episode of urinary tract infection 6 months back which was well-controlled with appropriate antimicrobial therapy based on culture sensitivity report. He is also a patient of Parkinson's disease and was on Levodopa. In April 2018, his hemoglobin (Hb) was 95 g/L with reticulocyte count of 1.2% and normal iron studies following which he was started on 40 microgram once weekly subcutaneous (SC) injection of rEPO (darbepoetin-α) at an outside hospital. He continued to be stable for the next 2 months while on darbepoetin-α following which he experienced increasing fatigue and tiredness. In June 2018, despite ongoing rEPO therapy, his Hb fell down to 85 g/L and then to 52 g/L, thus requiring weakly once packed red cell transfusion. His endoscopic evaluation was unremarkable and stool occult blood test was negative.
His complete blood count (CBC) prior to marrow evaluation at our center revealed the following: Hb 62 g/L; packed cell volume 18.8%; mean corpuscular volume 90 fL (ref. 80–98 fL); total leukocyte count 9.3 × 109/L (ref. 4–11 × 109/L) with a differential count of neutrophils 65%, lymphocytes 30%, and monocyte 05%; platelet count 170 × 109/L (ref. 150–450 × 109/L), and a corrected reticulocyte count 0.04%. Peripheral smear examination revealed normocytic normochromic anemia without any evidence of ovalomacrocytes, pseudo Pelger Heut or hypersegmented neutrophils, blasts, and adequate number of platelets. Biochemical analysis revealed serum iron of 94 μg/dL (ref. 35–150 μg/dL), total iron binding capacity 157 μg/dL (ref. 250–450 μg/dL), percentage saturation 60% (ref. 13%–45%), ferritin >2000 ng/mL (ref. 28–397 ng/mL), vitamin B12 499 pg/mL (ref. 211–911 pg/mL), creatinine 1.8 mg/dL, total protein 5.2 g/dL (ref. 6.4–8.2 g/dL), albumin 2.7 g/dL, globulin 2.5 g/dL, liver transaminases <50 U/L, alkaline phosphatase 92 U/L (ref. 50–135 U/L), calcium 8.5 mg/dL (ref. 8.6–10.3 mg/dL), and uric acid 5.3 mg/dL (ref. 3.5–7.2 mg/dL).
Bone marrow aspirate (BMA) and trephine biopsy revealed normocellular marrow for age with marked suppression of erythropoiesis (myeloid to erythroid ratio = 18:1) with relative prominence of early erythroid precursors suggestive of maturation arrest. The erythroid precursors together constituted 5% of all marrow nucleated cells in a 500-cell differential count on BMA. There was no evidence of any viral inclusions on early erythroid progenitors suggestive of Parvo B19 infection, nor was there any evidence of significant dyspoiesis in myeloid and megakaryocyte lineage. There was no evidence of any granuloma, lymphoma, clonal plasma cells, or metastatic malignancy. Glycophorin immunohistochemical staining, performed on trephine section, confirmed the near-total absence of erythroid islands (<10% of all nucleated cells in inter trabecular marrow spaces) [Figure 1a–c]. His serum immune fixation electrophoresis was negative for monoclonal gammopathy, and complete radiological examination was unremarkable. The CBC and marrow findings were consistent with a diagnosis of PRCA. Conventional G-banding cytogenetics from the marrow aspirate sample revealed a normal male karyogram (46, XY (20)). His serological profile was negative for viral infections such as EBV, CMV, HSV, Parvo B19 (both by polymerase chain reaction and ELISA), HIV, an hepatitis B and C viruses; and his autoimmune work up and Coombs test were all negative. His serum EPO level was found to be elevated at 53.20 mIU/mL (ref. 4.3–29 mIU/mL, chemiluminiscence immunosorbent assay), suggestive of EPO resistance. A diagnosis of darbepoetin-α-induced PRCA was made following which injection darbepoetin-α was stopped, and he was started on oral prednisolone (8 mg twice daily) along with transfusion support. Presently, his Hb is stable at 94 g/L while on prednisolone with no transfusion requirement. Anti-EPO antibody titer was not tested due to financial and technical constraints, and rEPO rechallenge was not done. The detailed sequence of events is depicted in Figure 2. The verbal informed consent was obtained from the next of the kin of the patient for this.

- (a and b) Hematoxylin–eosin-stained bone marrow trephine biopsy section from the case demonstrating normocellular marrow with morphologically normal maturing myeloid elements and megakaryocytes with near-total absence of erythroid islands (maturation arrest). (c) Note there is marked reduction in erythroid islands as highlighted by lack/absence of glycophorin staining by immunohistochemistry (peroxidase–antiperoxidase). Note the background RBCs in the section which serve as positive internal control for glycophorin (original magnification: a, ×200; b and c, ×400)
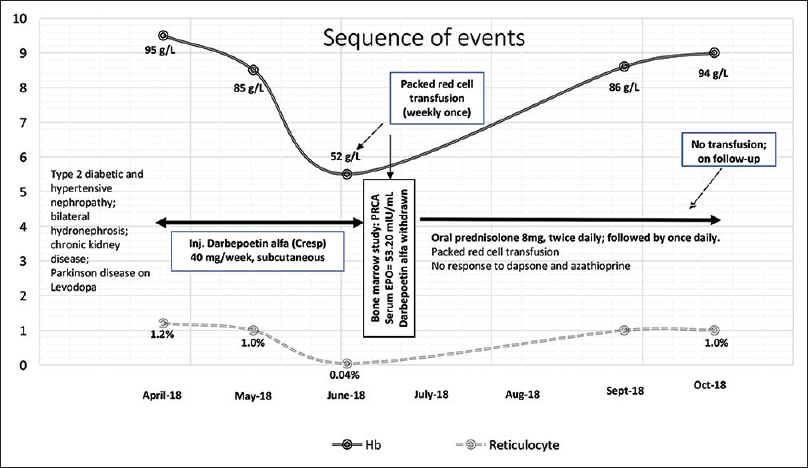
- Sequence of events in the present case
Discussion
rEPO-associated PRCA is a rare clinical syndrome characterized by the presence of following set of criteria as proposed by Casadevall et al.: (1) anemia with reticulocytopenia (<2 × 109/L) while on rEPO therapy with or without transfusion requirement due to erythroblastopenia in an otherwise morphologically normal bone marrow, (2) resistance to EPO therapy, and (3) presence of neutralizing anti-EPO antibody.[2] Antibodies to rEPO, detectable in serum of such patients, neutralize not only the exogenous erythropoietin stimulating agents but also the endogenous EPO, thus causing maturation arrest at the stage of early erythroid progenitors in the bone marrow.[8] Our patient satisfied the first two criteria, and anti-EPO antibody testing was not done.
rEPO-associated PRCA came into picture with surge of cohort of cases in mid-90s till 2004–2005 after the usage of reformulated [human serum albumin (HSA)-free, polysorbate 80, and glycine-rich] preparations of epoetin-α (Eprex/Erypo) through subcutaneous route, outside the United States.[29] As a result, by 2005, nearly 200 cases of rEPO-related PRCA cases were reported worldwide; European countries accounted for nearly 60% of all Eprex-related cases, whereas only four cases were reported from the United States due to usage of epoetin-α formulation [Epogen (Procrit)].[2] Sporadic cases of PRCA following usage of darbepoetin-α were also reported.[10]
Bennett et al. reviewed the follow-up outcome data of 170 of 191 rEPO-associated PRCA cases occurring in patients with CKD as reported by US Food and Drug Administration Research on Adverse Drug Events and Reports (RADAR) project.[9] The mean age of the subjects was 62 ± 17 years with a male preponderance (66% males vs. 34% females). A total of 169 cases had received rEPO via subcutaneous route, whereas one Epogen-treated patient received epoetin via intravenous route. The mean duration of usage of epoetin prior to development of PRCA was 9 months for Eprex, 18 months for NeoRecormon, and 24 months for Epogen. The hematologic recovery (following withdrawal of rEPO) with and without immunosuppressive therapy was highly significant (57% vs. 2%, respectively, P < 0.001), and nearly 95% had complete recovery following renal transplantation (n = 19 cases). Among 34 patients who received epoetin after the onset of PRCA, 56% regained epoetin responsiveness. Nearly 90% of the patients who did not have detectable anti-EPO antibodies in their serum achieved hematologic recovery following rechallenge with rEPO.[9] Among five cases (four males and one female; age range: 22–68 years) of rEPO-associated PRCA reported from India, four had elevated anti-EPO antibody demonstrated by radioimmunolabeled precipitation assay, two received Eprex, and one received Wepox (follow-on epoetin-α). Among four of them where detailed follow-up data were available, two had successful hematological recovery following immunosuppressive therapy and renal transplantation, one had a dramatic response to rituximab therapy, whereas rechallenge with a different rEPO (darbepoetin-α) along with immunosuppressive therapy lead to recovery in another individual [Table 1].[34567] Another case (data unpublished) from Pondicherry was noted in a 67-year-old female with CKD who received Eprex and finally succumbed to CKD-related complications.
| Authors, year, place | Age, gender | Indication | rEPO used | Route of admin., duration | Anti-EPO antibody, method | Serum EPO (ref. 10-30 mU/mL) | Management | Outcome |
|---|---|---|---|---|---|---|---|---|
| Bahadur et al.,[3] 2005, Mumbai | 22 years, male | CKD on HD | ND | SC, 3 months | 23% (ref. <4.7%), RIPA | Not tested | Cyclosporin + Pn; plasmapheresis, PRCT; renal transplantation | Stable disease, no rechallenge |
| Srinivas et al.,[4] 2007, New Delhi | ND | ND | ND | ND | ND | ND | ND | ND |
| Keithi-Reddy et al.,[5] 2008, Coimbatore | 57 years, female | CKD (CIN on HD) | Wepox (follow-on epoetin-α) | SC, 5 months | Positive, RIPA | 558 | Azathioprine + PRCT; renal transplantation | Recovery, 6 months post transplantation, no rechallenge |
| Ram et al.,[6] 2005/2013, Hyderabad | 53 years, male | CKD (HTN) on PD | Eprex | SC, 6 months | Positive, RIPA | Not tested | Pn+cyclosporine × 4 years (2002-2006); rechallenge with darbopoetin-α (2011) after anti-EPO antibody negativity | Recovery and stable disease |
| Mahajan et al.,[7] 2015, Chennai | 68 years, male | CKD (HTN) on HD | Eprex | IV followed by SC, 6 months | Positive, RIPA | 34.6 | Cyclophsphamide + Pn + PRCT; followed by rituximab | Recovery, 6 months post rituximab, no rechallenge. |
| Present case, 2018, Bhubaneswar | 76 years, male | CKD (T2DM + HTN) | Cresp (Darbopoetin-α) | SC, 2 months | Not tested | 53.20 | Az + dapsone; followed by methyl prednisolone + PRCT | Stable disease, no rechallenge, 8 months post withdrawal of rEPO |
CKD: Chronic kidney disease; ND: Not described; HD: Hemodialysis; CIN: Chronic interstitial nephritis; HTN: Systemic hypertension; PD: Peritoneal dialysis; CKD: Chronic kidney disease; T2DM: Type 2 diabetes mellitus; rEPO: Recombinant erythropoietin; SC: Subcutaneous; IV: Intravenous; RIPA: Radioimmunoprecipitation assay; Pn: Prednisolone; Az: Azathioprine; PRCT: Packed red cell transfusion. #Another case occurred in an elderly female patient with CKD who was receiving Eprex, but succumbed to CKD-related complications (data not published)
The pathophysiologic mechanism linked to rEPO-associated PRCA is complex and seems to be multifactorial. As hypothesized by Macdougall et al., the central event seems to be a breach in immunological tolerance resulting in increased antigen recognition by autoreactive T cells and subsequent B-cell-mediated antibody response in genetically susceptible (HLA-DRB1*9 positive) individuals.[11] The HSA, used in previous formulations, was less immunogenic due to its stabilizing effect on proteins, whereas polysorbate 80 and tungsten (in vials) in newer formulations help conformational changes in protein moiety causing more aggregation, leading to more antigenicity. The subcutaneous route of administration due to slower rate of absorption may also cause increased antigenic recognition and presentation by cutaneous Langerhan cells to autoreactive T cells. Also, SC route of administration offers self-administration and hence inappropriate handling at home. Advanced age, concurrent infections, intermittent illness, adjuvant therapies, comorbidities, and immune status of the individuals all might contribute to the immune dysregulation as was evident in the present case.[11]
To conclude, acquired PRCA following usage of rEPO formulations such as darbepoetin-α is possible, and this needs to be kept well in mind while managing patients with renal anemia and be evaluated thoroughly.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pure red cell aplasia. In: Kaushansky K, Lichtman MA, Prchal JT, Levi MM, Press OW, Burns LJ, eds. Williams Hematology (9th ed). New York, NY: McGraw-Hill Education; 2016. p. :539-48.
- [Google Scholar]
- Recommendations on hematological criteria for the diagnosis of epoetin-induced pure red cell aplasia. Eur J Hematol. 2004;73:389-96.
- [Google Scholar]
- Renal transplantation in a patient of end stage renal disease with anti-erythropoietin antibody induced pure red cell aplasia. Indian J Nephrol. 2005;15:22-5.
- [Google Scholar]
- Thirty-nine cases of pure red cell aplasia: A single center experience from India. Hematology. 2007;12:245-8.
- [Google Scholar]
- Ten year follow up of erythropoietin induced autoimmune pure red cell aplasia. Ind J Nephrol. 2013;23:323-4.
- [Google Scholar]
- Rituximab: A viable treatment option for epoetin-induced pure red cell aplasia. Indian J Nephrol. 2015;25:366-9.
- [Google Scholar]
- Detection of circulating anti-erythropoietin antibodies in patient on recombinant erythropoietin therapy. Indian J Nephrol. 2004;14:187-91.
- [Google Scholar]
- Long-term outcome of individuals with pure red cell aplasia and antierythropoietin antibodies in patients treated with recombinant epoetin: A follow-up report from the Research on Adverse Drug Events and Reports (RADAR) Project. Blood. 2005;106:3343-7.
- [Google Scholar]
- Antibody-mediated pure red cell aplasia in a dialysis patient receiving darbepoetin alfa as the sole erythropoietic agent. Nephrol Dial Transplant. 2006;21:2963-5.
- [Google Scholar]
- Antibody-mediated pure red cell aplasia in chronic kidney disease patients receiving erythropoiesis-stimulating agents: New insights. Kidney Int. 2012;81:727-32.
- [Google Scholar]







