Translate this page into:
Amyloid Proximal Tubulopathy and Amyloid Casts: An Unusual Finding in Multiple Myeloma
Address for correspondence: Dr. A. A. Kurien, Department of Pathology, Renopath Center for Renal and Urological Pathology, AL 190, 1st Street, 12th Main Road, Shanthi Colony, Anna Nagar, Chennai - 600 040, Tamil Nadu, India. E-mail: anila_abraham08@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Patients with multiple myeloma (MM) often develop renal manifestations. The majority of cases present as cast nephropathy, amyloid light-chain (AL) amyloidosis, and monoclonal immunoglobulin deposition disease. AL amyloidosis usually involves the glomeruli, blood vessels, and interstitium. It is extremely uncommon to find isolated intratubular deposition of AL amyloid. Our patient presented with rapid worsening of renal function due to isolated intratubular deposition of AL amyloid, where the biopsy revealed amyloid proximal tubulopathy and amyloid cast nephropathy. Our case provides new insights into the complicated pathophysiology of the abnormal light chains in MM. This case is, to our knowledge, the second case of amyloid proximal tubulopathy reported in literature.
Keywords
Amyloid cast nephropathy
amyloid proximal tubulopathy
amyloidosis
multiple myeloma
Introduction
Approximately half of the patients with multiple myeloma (MM) develop kidney injury during the disease.[1] Cast nephropathy, amyloid light-chain (AL) amyloidosis, and monoclonal immunoglobulin deposition disease (MIDD) are the common renal manifestations of MM. Less common manifestations include light-chain proximal tubulopathy, light-chain tubulointerstitial nephritis, and cryoglobulinemic glomerulonephritis.[2]
AL amyloidosis frequently involves the glomeruli, blood vessels, and interstitium. Isolated intratubular deposition of AL amyloid is extremely rare, with very few cases reported in literature.[345] We report the case of a patient with MM who presented with rapid deterioration of renal function due to intratubular deposition of AL amyloid, manifesting as amyloid proximal tubulopathy and amyloid cast nephropathy.
Case Report
A 62-year-old woman presented with easy fatigability for the past 3 months. She was a hypertensive on olmesartan 20 mg once a day for the past 5 years. She had no other significant medical history. On examination, she had pallor, pulse rate of 80/min, and blood pressure of 130/80 mm/Hg. Her cardiac examination was normal, lungs were clear, and abdomen was soft with no organomegaly. Urinalysis showed 4+ protein, 8–10 red cells/hpf; urine protein-creatinine ratio 4.2. Hemoglobin was 7.1 g/dl and hematocrit 20.9, total red blood cell count 2.3 million/mm3, total white blood cell count of 13,600 cells/mm3, platelet count 87,000/mm3. Peripheral smear revealed normocytic normochromic anemia and mild thrombocytopenia; blood urea 48 mg/dl; serum creatinine 3.1 mg/dl; prothrombin time 15.7 s; international normalized ratio 1.26; activated partial thromboplastin time 23.8 s; and direct Coomb's test negative. Serum total protein was 10.7 g/dl, with albumin of 3.3 g/dl and A:G ratio of 0.4:1. Serum protein electrophoresis showed total protein of 9.7 g/dl (6.4–8.2), albumin 3.57 g/dl (3.2–4.6), α1 globulin 0.3 g/dl (0.15–0.33), α2 globulin 0.79 g/dl (0.72–1.06), β globulin 0.93 g/dl (0.74–1.06), and γ globulin 4.11 g/dl (0.91–1.71). M-spike (2.78 g/dl) and myeloma band were detected. A bone marrow biopsy showed plasmacytosis (30%) including some bi-nucleate and tri-nucleate forms. Skeletal survey showed multiple punched out osteolytic lesions in the skull. Renal ultrasound showed normal-sized kidneys with normal corticomedullary differentiation. She received supportive treatment and was started on hemodialysis as her serum creatinine galloped to 9.2 mg/dl.
A renal biopsy was performed. Light microscopy had eight glomeruli for evaluation. No diffuse or nodular mesangial expansion was noted. No spikes or double contours were seen on the glomerular basement membranes. No endocapillary proliferation, fibrinoid necrosis or crescent formation was present. Tubular epithelial cells showed signs of acute injury, with flattened epithelium and loss of brush borders. About 20% of the tubules had amorphous periodic acid-Schiff (PAS) negative casts. Occasional casts were rimmed by inflammatory cells. Some casts had spicules at the periphery and were PAS and silver positive and blue on Masson's trichrome stain. The spicules and periphery of these casts were Congo red positive, exhibiting Apple-green birefringence upon polarization which is diagnostic of amyloid [Figure 1]. Few of the proximal tubules had small, round amorphous bodies in the cytoplasm which was PAS negative, weakly silver positive, and blue on trichrome stain [Figure 2]. These bodies were also Congo red positive [Figure 3]. Interstitium was edematous and was infiltrated by lymphocytes and a significant number of eosinophils. There was mild fibrous intimal proliferation of the arteries. Congo red stain was negative in the glomeruli, interstitium, and blood vessels.
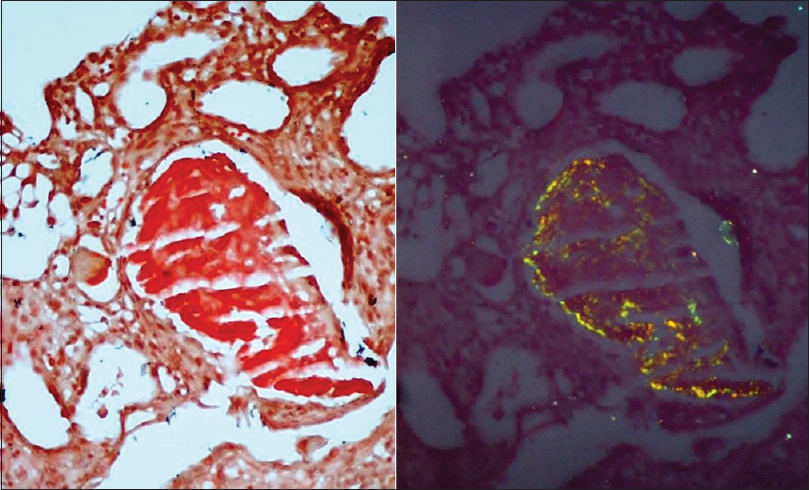
- The tubular casts are Congo red positive under white light (left) and polarized light (right)
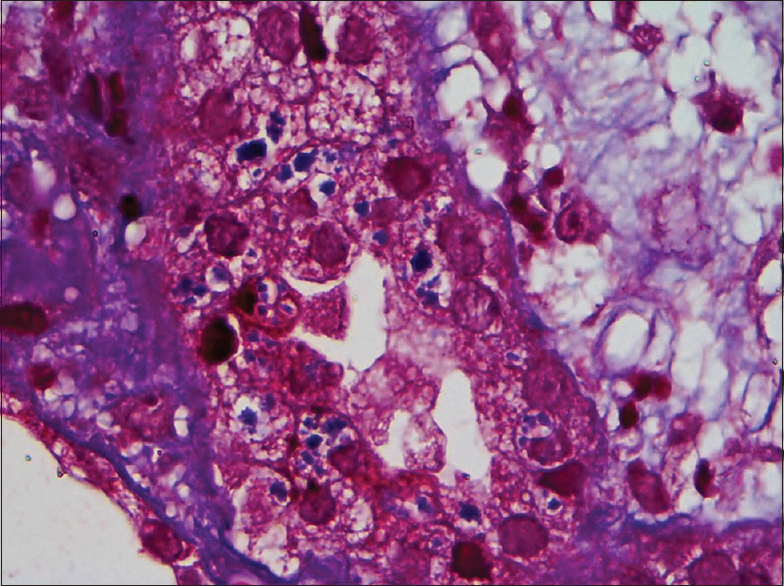
- Proximal tubular epithelial cells have small round amorphous bodies in the cytoplasm which is blue on trichrome stain
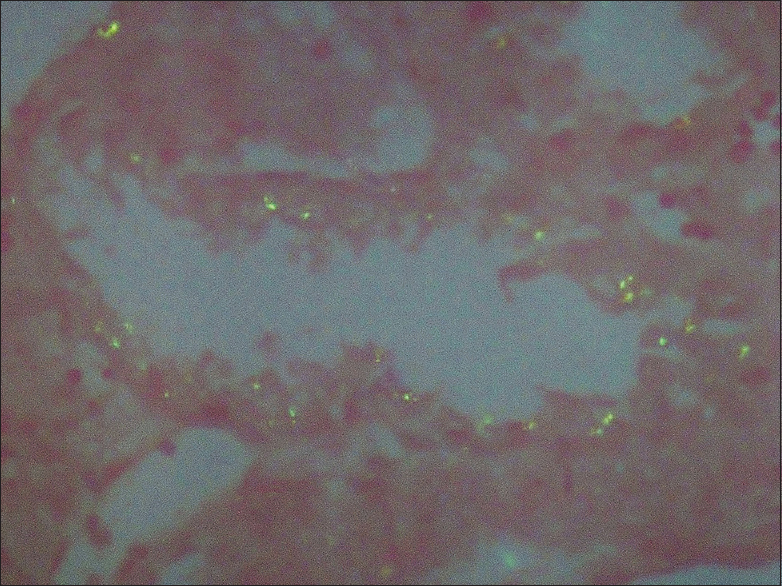
- These bodies are Congo red positive, under polarized light
The tissue submitted for immunofluorescence study was stained for IgG, IgM, IgA, C3, C1q, and kappa and lambda light chains. There was intense (+3) staining for lambda light chain over the tubular casts and the intracellular round bodies [Figure 4]. Kappa light chain was negative [Figure 5]. The tubular basement membranes were negative for kappa and lambda light chains. Immunoglobulins and complement were also negative. The renal biopsy was diagnosed as amyloid cast nephropathy and amyloid proximal tubulopathy.
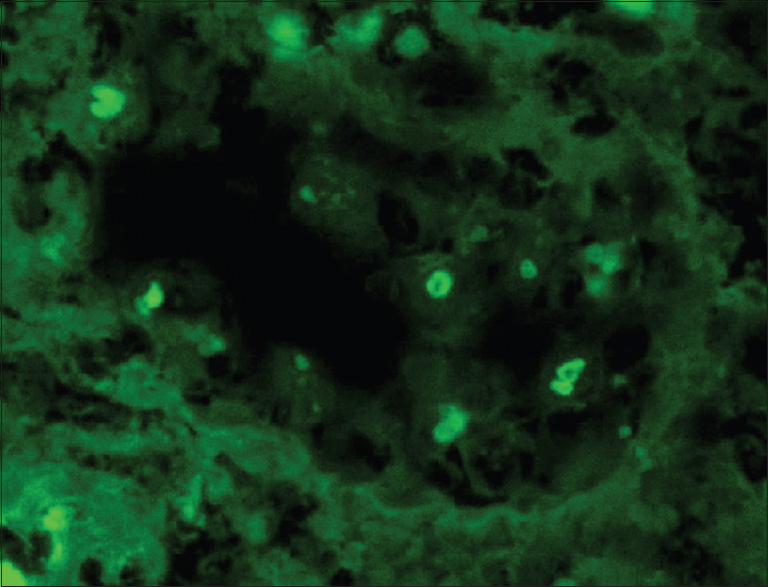
- Intense staining for lambda light chain over the intracellular round bodies
- Kappa light chain is negative over these intracellular round bodies
She was treated with bortezomib 2 mg/m2/dose along with oral dexamethasone 40 mg weekly. She required five sessions of hemodialysis and urine output improved. Creatinine stabilized at 2.8 mg/dl. Her 2-month follow-up serum creatinine was 2.7 mg/dl after five doses of bortezomib. However, proteinuria persisted.
Discussion
MM is characterized by the proliferation of clonal B-cells which produce abnormal immunoglobulin, usually abnormal kappa or lambda free light chains (FLCs). In some patients, the abnormal light chain produced can become amyloidogenic and this is known as AL amyloid.[1]
Acute kidney injury is an important cause of morbidity and mortality in patients with myeloma. Light-chain proximal tubulopathy, light-chain tubulointerstitial nephritis, and cast nephropathy occurring in isolation or together are the main causes. Kidney injury in a patient with myeloma can also be due to nonlight-chain-related mechanism. These include hypercalcemia, uric acid nephropathy, sepsis, volume depletion, rhabdomyolysis, and drug toxicity.[2]
The organs most frequently involved in AL amyloidosis are the kidney and heart; however, any tissue other than the brain can be involved.[6] There was no other apparent organ involvement in our patient. In the kidney, the predominant site of deposition of the amyloid fibrils is the glomeruli presenting as nephrotic syndrome with progressive worsening of renal function. In approximately 10% of patients, amyloid deposition occurs in the interstitium or renal vasculature presenting as renal dysfunction without significant proteinuria.[7] It is not uncommon to find amyloid deposits in more than one renal compartment. Amyloid can also rarely be deposited in the tubular lumen.[34] Demonstrating amyloid within the tubular epithelial cells is extremely rare with only one case report published in literature.[5]
AL amyloidosis is diagnosed when amyloid is demonstrated in the tissue. The amyloid deposits shows apple-green birefringence when stained with Congo red and viewed under polarized light and the immunofluorescence shows staining for either kappa or lambda light chain (light-chain restriction).[6]
The mechanism of amyloid formation within the tubules is not fully understood. The FLCs have a low molecular weight (20–25 kDa) and they are filtered through the glomerular capillary walls. Amyloid fibrils being much larger in size are not filtered. The proximal tubular epithelial cells endocytose the light chain using the megalin/cubilin receptor present in the brush borders. They are then catabolized by the endosomal-lysosomal pathway.[8] The physicochemically abnormal light chains get accumulated in the lysosomes as they cannot be effectively catabolized. Lysosomes eventually breakdown, with spillage of their contents into the cytoplasm causing cell damage. The FLCs that reach the distal tubule interact with uromodulin and form light-chain casts.[2]
The structural abnormalities of the FLC as well as environmental factors such as pH and urea concentrations determine their pathogenic effects. Some FLCs have the propensity to crystallize within the lysosomes giving rise to Fanconi syndrome.[9] Very rarely, as in our patient, the FLC undergoes conformational changes and acquires the characteristics of AL amyloid. The amyloid formed within the tubular epithelial cells is secreted into the tubular lumen where they form amyloid casts.[34] Our case demonstrated the presence of Congo red positive amyloid bodies within the tubular epithelium as a well as amyloid casts.
To summarize, our patient had an extremely rare presentation of intratubular amyloid deposition. Our case suggests that the abnormal light chains filtered by the Glomerular basement membrane, under certain unknown circumstances, can undergo conformational changes within the tubular epithelial cells and produce amyloid casts. This case adds to the diversity and complexity of the pathophysiology of the abnormal light chains in MM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Renal manifestations of plasma cell dyscrasias: An appraisal from the patients' bedside to the research laboratory. Ann Diagn Pathol. 2000;4:174-200.
- [Google Scholar]
- Amyloid proximal tubulopathy: A novel form of light chain proximal tubulopathy. Clin Kidney J. 2012;5:130-2.
- [Google Scholar]
- Prevalence and origin of amyloid in kidney biopsies. Am J Surg Pathol. 2009;33:1198-205.
- [Google Scholar]
- Low molecular weight proteins and the kidney: Physiologic and pathologic considerations. Ultrastruct Pathol. 1994;18:89-98.
- [Google Scholar]
- The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2011;8:43-51.
- [Google Scholar]







