Translate this page into:
Aquaporins: The renal water channels
Address for correspondence: Dr. Sanjay K. Agarwal, Department of Nephrology, All India Institute of Medical Sciences (AIIMS), New Delhi-110 029, India. E-mail: skagarwal58@yahoo.co.in
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Water is the most abundant molecule in any cell. Specialized membrane channel, proteins called aquaporins, facilitate water transport across cell membranes. At least seven aquaporins (AQP): 1, 2, 3, 4, 6, 7, and 11 are expressed in the kidneys. Aquaporins play a role in both the short-term and long-term regulation of water balance as well as in the pathophysiology of water balance disorders. Aquaporin is composed of a single peptide chain consisting of approximately 270 amino acids. Inherited central and nephrogenic diabetes insipidus are primarily due to the decreased expression of AQP2 while mutation in the AQP2 molecule is responsible for inherited central diabetes insipidus. In acquired causes of nephrogenic diabetes insipidus, there is a downregulation of AQP2 expression in the inner medulla of the kidney. Nephrotic syndrome is characterized by excessive sodium and water reabsorption, although in spite of this, patients do not develop hyponatremia. There is a marked downregulation of both AQP2 and AQP3 expression, which could be a physiologic response to extracellular water reabsorption in patients with nephrotic syndrome. There are some conditions in which aquaporin expression has been found to increase such as experimentally induced heart failure, cirrhosis, and pregnancy. Some drugs such as cisplatin and cyclosporine, also alter the expression of aquaporins. The three-pore model of peritoneal transport depicts the importance of aquaporins. Thus, the understanding of renal water channels has solved the mystery behind many water balance disorders. Further insights into the molecular structure and biology of aquaporins will help to lay a foundation for the development of future drugs.
Keywords
Aquaporins
diabetes insipidus
kidney
water channels
Water is the most abundant molecule in a cell. Although the plasma membrane separates the interior of the cell from its extracellular environment, specialized membrane channels facilitate water transport across these biomembranes. Such water channels, which are in the form of proteins, are called aquaporins. In bacteria, plants, and animals, these channel proteins are very conserved, however, there are many human isoforms. At least seven aquaporins (AQP): 1, 2, 3, 4, 6, 7, and 11 are expressed in the kidneys [Table 1]. Aquaporins play a role in the short-term and long-term regulation of water balance and also in the pathophysiology of water balance disorders.
| Aquaporin group | Localization in kidney |
|---|---|
| AQP 1 | APM/BLM of proximal tubules and descending thin limbs |
| AQP 2 | APM/VES of principal cells of collecting ducts |
| AQP 3 | BLM of collecting ducts |
| AQP 4 | BLM of medullary collecting ducts |
| AQP 6 | Cortex, Medulla |
| AQP 11 | Proximal tubule (Intracellular) |
AQP: Aquaporin, APM: Apical membrane, BLM: Basolateral membrane, VES: Vesicles
Discovery
Serendipity played a major role in the discovery of aquaporins. A 28 kDa polypeptide was noted during the biochemical purification of the 32 kDa core polypeptide of red cell Rh blood group antigen. The protein was found to be a tetramer and was functionally related to the major intrinsic protein of the lens.1 Abundance of the 28 kDa polypeptide in highly water permeable tissues, red cells, renal proximal tissues, and descending thin limbs led the late John C. Parker at the University of North Carolina at Chapel Hill to predict that it was probably a water channel. Peter Agre named the protein “aquaporin”.2
Structure
Aquaporin is composed of a single peptide chain consisting of approximately 270 amino acids. The deduced amino acid sequence of AQP1 predicted six membrane-spanning domains with intracellular amino (N) and carboxy (C) termini. There are three extracellular loops (A, C, and E) and two intracellular loops (B and D). Highly conserved regions are present within loops B and E, each of which contains the consensus motif, asparagine-proline-alanine (NPA). When these two loops are folded into the lipid bilayer and surrounded by transmembrane domains, they may form a hydrophilic path for the water transfer through the lipid bilayer. This model is called the “hourglass model” [Fig. 1].3 Fourier transform infrared spectroscopy was used to further characterize the secondary structure of AQP1 and the results revealed that six closely associated alpha helices span the lipid membrane.4 Moreover, the 3D structure of AQP1 was determined at 6Å resolution by cryoelectron microscopy. Each AQP1 monomer has six-tilted, bilayer-spanning alpha helices, which form a right-handed bundle surrounding a central density. These studies also confirmed the organization of a tetrameric complex in the membrane.5

- Structure of aquaporin 1
Aquaporin 1
The human aquaporin gene is located on chromosome 7p14. It has a strong homology with the major intrinsic protein of the bovine lens (MIP, AQP0) and was named initially as “channel-like integral protein of 28 kD”(CHIP-28) and later, Aquaporin 1 (AQP1). AQP1 is present in the apical and basolateral surfaces of the proximal convoluted tubules, the descending thin limbs of the loop of Henle, and in the nonfenestrated endothelium of the descending vasa recta.6 The localization of AQP1 is in concordance with the known water permeability characteristics of the kidney, supporting the hypothesis that AQP1 is mainly responsible for osmotic permeation in the regions of the kidney. It comprises 3.8% of the isolated proximal tubule brush border protein. As AQP1 is absent in the collecting duct where water absorption is regulated by the antidiueretic hormone (ADH), AQP1 must be responsible for constitutive water reabsorption.
In order to analyze the role of AQP1 in water absorption for urine formation, AQP1 null mice were generated by targeted gene disruption. The osmotic water permeability in the proximal tubule membrane vesicles is reduced by eight-fold compared to wild mice.7 This shows that AQP1 is indispensable for efficient urine concentration, which is further confirmed by the partial correction of the urine-concentrating defect in AQP1 null mice by introducing the AQP1 via adenovirus-mediated gene delivery. These results also establish the cellular mechanism of water reabsorption: water passes through the epithelial layer not paracellularly, but transcellularly via the aquaporins. The Colton blood group antigen was identified to be an AQP1 in human RBCs. Individuals lacking the Colton antigen showed AQP1 gene nonsense / missense mutations, although they did not suffer from any clinical abnormalities.8 Recently, defective urinary-concentrating ability was shown in an individual completely lacking AQP1.9
Aquaporin 2
Aquaporin 2 (AQP2) was cloned as an ADH-regulated water channel of kidney collecting ducts (CD) in rats and humans; it is present in the principal cells of the CD. In the basal state, AQP2 is stored in intracellular vesicular compartment but upon ADH stimulation, it rapidly moves to the apical membrane where it acts as a water channel for the concentration of urine. Regulation of AQP2 trafficking is shown in Fig. 2. Vasopressin acts at V2 receptors in the basolateral plasma membrane (BLM) of CD principal cells. Activation of adenyl cyclase accelerates the production of cyclic AMP from ATP; the cyclic AMP then binds to the regulatory subunit of protein kinase A (PKA) which activates the catalytic subunit of PKA. PKA then phosphorylates AQP2 in the intracellular vesicles and possibly other cytosolic or membrane proteins. Microtubular motor proteins and vesicle targeting receptors (VAMP-2, Syntaxin-4, and NSF) may participate in the specificity of AQP2 targeting to the apical membrane to increase water permeability. Cyclic AMP also participates in the long-term regulation of AQP2 by increasing the levels of the catalytic subunit of PKA in the nuclei, which phosphorylates transcription factors such as cAMP responsive element-binding protein (CREB-P) and c-jun / c-fos. Binding of these proteins is thought to increase the gene transcription of AQP2 resulting in synthesis of AQP2 protein, which in turn, enters the regulated trafficking system.10
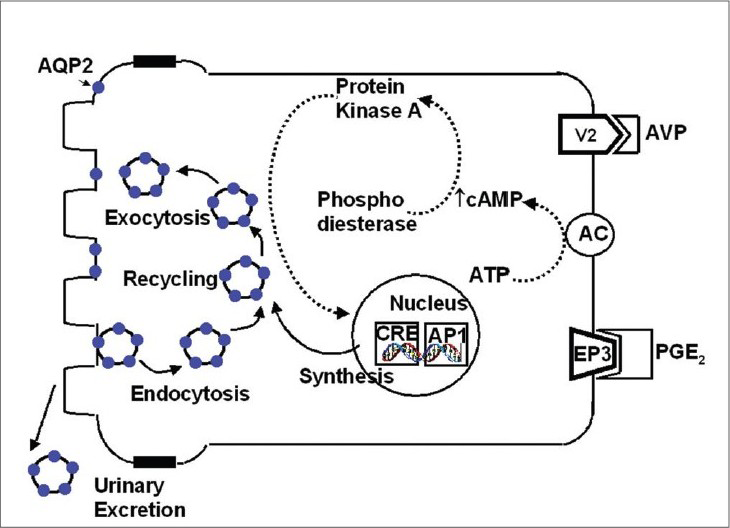
- Regulation of aquaporin 2 in principal cell
Aquaporin 3
Aquaporin 3 (AQP3) is localized along the BLM of the principal cells of the collecting ducts. Along with AQP2, it aids in the exit of water from the cell into the interstitium for transepithelial transfer of water across CD cells. It permits transport of both water and glycerol and there is a possibility of separate water- and glycerol-transporting domains. Due to its ability to mediate glycerol transport, AQP3 was also called the glycerol intrinsic protein [GLIP]. Although the role of AQP3 in water transport is not fully understood, an AQP3 knockout experiment provided a clue. The growth and phenotype of AQP3 null mice were grossly normal except for polyuria. These animals consumed ten times more fluid and excreted urine of low osmolality.11 No humans have yet been identified with AQP3 deficiency.
Aquaporin 4
Aquaporin 4 (AQP4) together with AQP3 is localized at the BLM of the principal cells of CDs. It may serve as a water channel for the exit of water from the BLM for the concentration of urine. AQP3/AQP4 double knockout mice show greater impairment of urine-concentrating ability than AQP3 single knockout mice.11
Aquaporin 6
Aquaporin 6 (AQP6) is expressed in the acid-secreting intercalated cells of the collecting ducts in the kidney. It co-localizes with H+ ATPase, suggesting that low pH could activate the protein. Intracellular vesicles in the α-intercalated cells are thought to permit renal acid secretion by generating hydrochloric acid. To maintain electroneutrality, a pathway for anion secretion must exist and ClC-5 is located at this site. However, this protein is inactivated at low pH. Thus, the role of AQP6 in the α-intercalated cells may be to function as a chloride channel during the later stages of acid secretion.12
Aquaporin 11
Although aquaporin 11 (AQP11) can be identified on the basis of the unusual pore forming asparagine-proline-alanine (NPA) consensus motif, its function is unknown. It is localized intracellularly in the proximal tubule. AQP11 null mice exhibited vacuolization and cyst formation in the proximal tubule. AQP11 null mice were born normally but died before weaning due to advanced renal failure with polycystic kidneys. Further culture revealed an endosomal acidification defect in AQP11 null mice. This illustrates that AQP11 is essential for proximal tubular function.13
Pathophysiologic Roles of Aquaporins in Renal Disorders [Table 2]
| Conditions with reduced AQP2 expression and polyuria |
| Central diabetes insipidus |
| Aging |
| Lithium |
| Low-protein diet |
| Hypokalemia |
| Hypercalcemia |
| Postobstructive nephrogenic diabetes insipidus |
| Ischemia-induced acute kidney injury |
| Experimental chronic renal failure |
| Conditions with increased AQP2 expression and water retention |
| Cardiac failure |
| Pregnancy |
| Cirrhosis |
AQP2: Aquaporin 2
Inherited central and nephrogenic diabetes insipidus
Central Diabetes insipidus (CDI) is characterized by very low or undetectable levels of vasopressin. Using vasopressin-deficient Brattleboro rats as a model, it was demonstrated that AQP2 expression levels were markedly lower than those in the parent strains of Long Evans rats.14 Furthermore, prolonged treatment with either vasopressin or dDAVP results in marked increases in AQP2 expression and in apical plasma labeling in both the inner medulla and the cortical collecting ducts. Thus, dysregulation of AQP2 due to the absence of vasopressin plays a major role in the development of polyuria. In nephrogenic Diabetes insipidus (NDI), there is insensitivity to vasopressin in renal tubular cells.
90% of the inherited types are X-linked recessive due to mutations in the V2 receptor genes. The rest are either autosomal dominant or autosomal recessive mutations in the AQP2 gene located on chromosome 12q13.15
In the autosomal recessive type, mutations are mostly between the 5th and 6th transmembrane domains of the molecule. Two types of mutations have been identified: T125M or G175R with normal trafficking to plasma membrane but leading to the loss of water channel function, and another one that causes misrouting of the mutated AQP2 molecule to the endoplasmic reticulum leading to a failure to reach the plasma membrane. This misfolding can be corrected by chemical chaperones. In the autosomal dominant form, mutations are found in the C-terminus of AQP2. This mutated AQP2 is localized in aberrant intracellular compartments such as the Golgi apparatus, lysosomes, or BLM. Here, trafficking of AQP2 to the plasma membrane is impaired, but its fundamental 3-D structure is not affected by these mutations.1617
Acquired causes of nephrogenic diabetes insipidus
Hypokalemia and hypercalcemia may be associated with polyuria as a result of a defect in the renal response to ADH to maximally concentrate urine. A potassium-deficient diet fed to rats for 11 days caused hypokalemia-related nephrogenic diabetes insipidus (NDI) and seven days of a vitamin D-rich diet caused hypercalcemia-related NDI. There was downregulation of AQP2 expression in the inner medulla in both these studies.1819 Chronic lithium treatment resulted in a severe concentration defect and in the rat model, lithium caused a marked reduction in AQP2 levels in parallel with the development of severe polyuria. Urine volume increased three-fold after one week of lithium treatment and > six-fold after two weeks while AQP2 expression decreased to 58% of that in control rats at one week and to 33% at two weeks of lithium treatment. With the aid of proteomics, 77 different proteins were identified within the inner medullary collecting duct that were affected directly or indirectly by lithium treatment.20 Another cause of acquired NDI is postobstructive diuresis. Bilateral ureteral obstruction in a rat model showed that AQP2 levels were reduced to ¼th of the control levels. Even one week after the urine output had become normal, animals showed only ½ of their normal AQP2 levels. Secondly, they could not concentrate urine in response to dehydration as effectively as sham-operated rats.21
A low-protein diet also causes a urine concentration defect. In rats fed on a low-protein (8%) diet for two weeks, AQP2 expression decreased significantly in the inner medulla with a subsequent decrease in ADH-stimulated osmotic water reabsorption.22 Aging decreases the ability to elevate ADH levels in response to dehydration. A study revealed that 72 hours of water deprivation in four month- and 30 month-old rats resulted in increased AQP2 levels in only the younger rats.23
Aquaporin dysregulation in renal diseases
Nephrotic syndrome is characterized by excessive sodium and water reabsorption, but in spite of this, patients do not develop hyponatremia. There is marked downregulation of AQP2 and AQP3 expression, which could be a physiological response to extracellular water reabsorption.24 In ischemic ARF, structural changes are seen in the S3 segment of the proximal tubule and the thick ascending limb. Recently, Fernandez-Llama showed that CD water channels, AQP2, 3, 4 as well as proximal tubule AQP1 expression levels were significantly decreased in association with polyuria in response to ischemia. This suggests that CD is also affected by ischemia and that the polyuria seen in ARF may be partially due to reduced CD aquaporin levels.25 Similarly, experimental CRF induced by 5/6th nephrectomy is characterized by polyuria and ADH-resistant downregulation of AQP2 and 3 levels.26
Normally aquaporin is present in minimal amounts in the glomerulus. Immunohistochemistry of biopsy samples showed marked upregulation of AQP1 in the glomeruli in various renal disorders. Bedford et al. postulated that renal injury produced increased stress on cell integrity and increased aquaporin expression is an adaptive response to this stress.27 In contrast, they found a reduction in the expression of AQP2 and 3 in lesions with substantial interstitial fibrosis and reduced nephron numbers.
Conditions with increased aquaporin expression
Heart failure experimentally induced by left coronary artery ligation in rats showed that there was marked increase in AQP2 mRNA expression and a redistribution of AQP2 in the collecting ducts with increased targeting to the apical membrane. This regulation was seen only in rats with severe CHF (increased LVEDP and decreased serum sodium) but not in rats with compensated failure (increased LVEDP and normal serum sodium). Cirrhosis is another condition with variable AQP2 expression. Chronic intraperitoneal administration of carbon tetrachloride results in increased AQP2 mRNA and protein in rats. The V2 receptor antagonist, OPC 31260 reversed this increase. However, compensated cirrhosis induced by ligation of the common bile duct resulted in decreased AQP2 expression and consequently, V2 receptor antagonists were not effective. Another condition is pregnancy where rats had increased AQP2 levels in the first trimester that persisted throughout pregnancy; increased AQP2 trafficking was also seen. V2 receptor antagonists were effective in reducing AQP2 levels to those seen in the nonpregnant state.28
Aquaporins and drugs
In a controlled study, cisplatin (8 mg/kg) was injected intraperitoneally into male Sprague-Dawley rats. Four days later, the expression of AQP1, AQP2, and AQP3 proteins was determined in the kidney. Cisplatin treatment caused a polyuric renal failure in association with decreased free water reabsorption. Expression of AQP1 and AQP2 was decreased in the cortex and the outer and inner medulla, whereas that of AQP3 was decreased in the outer and inner medulla.29 In another study, rats were treated daily for four weeks with vehicle (VH; olive oil, 1 mL/kg sc) or cyclosporine, CsA (15 mg/kg sc). Increased urine volume, fractional excretion of sodium, decreased urine osmolality, and free-water reabsorption was seen in the CsA group as compared to the VH-treated rats. There was a decrease in the expression of AQP (1–4) and the urea transporter, UT (UT-A2, -A3, and UT-B). This shows that calcineurin is required for normal intracellular trafficking of AQP2; loss of calcineurin protein or activity disrupts AQP2 function.30
Aquaporins and peritoneal dialysis
The three-pore model of peritoneal transport depicts the importance of aquaporins. It assumes the capillary endothelium to be the major barrier to solute and water transport occurring through a system of pores that are classified into three broad categories: ultrasmall, small, and large pores. The abundant small pores (40 to 60 Å radii) are the tortuous intercellular clefts between the endothelial cells; they are responsible for small solute transport. The ultrasmall pores (radius 3 to 5 Å), also present in large numbers, are probably the transendothelial aquaporin-1. Solute free-water transport occurs across them by way of crystalloid osmosis. In addition, a few large pores (200 to 300 Å radii) are present, the nature of which is not well known; macromolecules such as albumin are transported across them [Fig. 3].
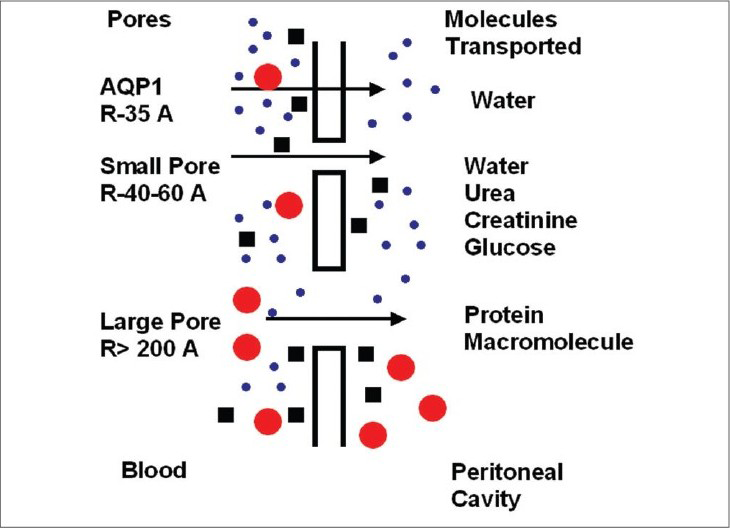
- Three-pore model of peritoneal transport
It is assumed that 40–50% of osmotic-induced ultrafiltration is mediated by aquaporins. As free water is transported through these pores, a drop in dialysate sodium concentration is expected to occur during the initial 60 to 90 minutes of the dwell. This phenomenon is known as sodium sieving. A dialysate/plasma sodium ratio of >0.88 correlates with an aquaporin deficiency at 60 minutes during the modified peritoneal equilibrium test.
Conclusion
The recognition and understanding of renal water channels has solved the mystery of many water balance disorders. Further insights into the molecular structure and biology of aquaporins will lay a foundation for the development of future drugs.
Source of Support: Nil
Conflict of Interest: None declared.
References
- The major intrinsic protein (MIP) of the bovine lens fiber membrane: Characterization and structure based on c DNA cloning. Cell. 1984;39:49-59.
- [Google Scholar]
- Molecular structure of the water channel through Aquaporin CHIP: The hourglass model. J Biol Chem. 1994;269:14648-54.
- [Google Scholar]
- Secondary structures comparison of aquaporin-1 and bacteriorhodopsin: A Fourier transform infrared spectroscopy study of two- dimensional membrane crystals. Biophys J. 1997;73:406-17.
- [Google Scholar]
- CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993;120:371-83.
- [Google Scholar]
- Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273:4296-9.
- [Google Scholar]
- Mutations in aquaporin-1 in phenotypically normal humans without functional CHIP water channels. Science. 1994;265:1585-7.
- [Google Scholar]
- Defective urinary-concentrating ability due to a complete deficiency of aquaporin-1. N Engl J Med. 2001;345:175-9.
- [Google Scholar]
- Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol. 1999;10:647-63.
- [Google Scholar]
- Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci USA. 2000;97:4386-91.
- [Google Scholar]
- Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol Cell Biol. 2005;25:7770-9.
- [Google Scholar]
- Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad USA. 1994;91:8984-8.
- [Google Scholar]
- Aquaporin-2, a vasopressin sensitive water channel, and nephrogenic diabetes insipidus. Intern Med. 1998;37:215-7.
- [Google Scholar]
- An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J Clin Invest. 1998;102:57-66.
- [Google Scholar]
- Hypokalemia induced downregulation of aquaporin-2 water channel expression in rat kidney medulla and cortex. J Clin Invest. 1996;97:1960-8.
- [Google Scholar]
- Vasopressin- elicited water and urea permeabilities are altered in IMCD in hypercalcemic rats. Am J Physiol. 1998;274:F978-85.
- [Google Scholar]
- Protemic analysis of lithium-induced nephrogenic diabetes insipidus: Mechanisms for aquaporin2 down regulation and cellular proliferation. Proc Natl Acad Sci USA. 2008;105:3634-9.
- [Google Scholar]
- Bilateral ureteral obstruction down regulates expression of vasopressin-sensitive AQP-2 water channel in rat kidney. Am J Physiol. 1996;270:F657-68.
- [Google Scholar]
- Changes in aquaporin-2 protein contribute to the urine concentrating defect in rats fed a low-protein diet. J Clin Invest. 1996;97:2807-14.
- [Google Scholar]
- Effect of aging in vasopressin and aquaporin responses to dehydration in Fischer 344-brown-Norway F1 rats. Am J Physiol. 1997;273:R35-40.
- [Google Scholar]
- Reduced renal water channel expression in puromycin aminonucleoside-induced nephritic syndrome. J Am Soc Nephrol. 1997;8:15-24.
- [Google Scholar]
- Decreased abundance of collecting duct aquaporins in post ischemic renal failure in rats. J Am Soc Nephrol. 1999;10:1658-68.
- [Google Scholar]
- Reduction of deep nephrons and the terminal collecting duct to a reduction in renal mass. Am J Physiol. 1979;236:F454-64.
- [Google Scholar]
- Aquaporin expression in normal human kidney and in renal disease. J Am Soc Nephrol. 2003;14:2581-7.
- [Google Scholar]
- Body water homeostasis: Clinical disorders of urinary dilution and concentration. J Am Soc Nephrol. 2006;17:1820-32.
- [Google Scholar]
- Cisplatin decreases the abundance of aquaporin water channels in rat kidney. J Am Soc Nephrol. 2001;12:875-82.
- [Google Scholar]
- Long-term treatment with cyclosporine decreases aquaporins and urea transporters in the rat in kidney. Am J Physiol Renal Physiol. 2004;287:F139-51.
- [Google Scholar]







