Translate this page into:
Clinical features, epidemiology, and short-term outcomes of proliferative lupus nephritis in Eastern India
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Race and ethnicity are important predictors of prognosis in lupus nephritis. This study was conducted to determine the clinical features, epidemiological profile, and short-term outcomes in patients of lupus nephritis from a single center in Eastern India. A total of 86 patients of class III/IV lupus nephritis were studied. Seventy-eight of them received cyclophosphamide for induction and eight of them received mycophenolate. The patients were evaluated for response, estimated glomerular filtration rate (eGFR), and proteinuria at 6 months. About 44% patients had a partial or complete response at 6 months and 64% at 1 year. The factors correlating with response at 6 months were older age at diagnosis, hypertension, activity, and chronicity indices and duration of symptoms prior to therapy. Chronicity index and hypertension were the predictors of response by logistic regression at 6 months. Compared to the Caucasian and African American patients, patients with proliferative lupus in Eastern India presented with a lower eGFR, lower proteinuria, and higher chronicity scores. Older age at diagnosis, hypertension, activity, chronicity indices, and duration of symptoms correlated with response. Short-term outcomes were similar to those described in Caucasian patients.
Keywords
Lupus nephritis
prognostic markers
race
remission
short term
Introduction
Renal involvement is a serious complication of systemic lupus erythematosus, and proliferative lupus nephritis (WHO classes III and IV) has poor outcomes.[1] With the advent of induction therapy with cyclophosphamide, the survival of patients has improved, with 5-year survival for class IV lupus nephritis at 82%.[2] 25-30% of patients of diffuse proliferative lupus nephritis will reach end-stage renal disease (ESRD) over 20 years of follow-up.[2]
Race has been shown to predict outcomes in lupus nephritis. Black patients have an increased frequency of lupus nephritis and worse survival rates.[34] Hispanic patients have increased disease activity, and 6-year renal survival is only 50%.[4] Studies on Asian patients suggested that they have an increased risk of developing lupus nephritis,[5] although the long- term outcomes are similar to white patients.[6] These reports are from an Oriental population; patients from South Asia were not well represented. A cohort of SLE patients from Pakistan was found to have a 5-year survival of 80%. Organ damage (including nephritis) was present in 76% of the patients in this cohort.[7]
The other factors which determine the outcomes are male gender, younger age, higher baseline creatinine, low socioeconomic status, nephrotic syndrome at presentation, resistant nephritic syndrome, severe anemia, hypertension, low levels of complement, anti-phospholipid antibodies, class IV histology, high activity and chronicity index, and treatment type.[58–11]
Chronic changes that occur as a result of delay in diagnosis and therapy are an important cause for failure to remit.[12] Patients who fail to achieve remission are at a higher risk of flares, (and flares of greater severity), and worse patient and renal survival. Patient survival is 95% in those who achieve remission, and 60% in those who do not; renal survival is 94% and 31% respectively.[10] Patients who achieve a partial remission have a six-fold higher risk of relapse than those who achieve complete remission.[6]
In the current study, we retrospectively analyzed a cohort of lupus patients who had diffuse proliferative lupus nephritis, and presented to the Department of Nephrology in the Institute of Postgraduate Medical Education and Research, Kolkata, India, between 2008 and 2010. These patients were treated with an induction protocol of IV pulse cyclophosphamide monthly for at least 6 months. Prognostic factors for response were assessed by both correlation and regression.
Materials and Methods
A total of 86 patients of class III/IV lupus nephritis,[13] had attended the OPD of the department of Nephrology from June 2008 to June 2010. All the patients were followed-up for more than 6 months. Patients who were deemed to be in ESRD and those in whom 6 months of therapy could not be administered (due to infection, development of ESRD or death) were excluded. All ages were considered for inclusion. Active lupus nephritis was defined by urine RBC >5/hpf or RBC/WBC/granular casts in the urine, proteinuria of more than 0.5 gm/day, and biopsy-proven renal disease. Renal biopsies were categorized according to WHO/ISN/RPS classification and activity and chronicity indices were noted.[14]
All the patients received induction therapy with intravenous pulse methylprednisolone (1000 mg once daily for three days). Seventy-eight patients received induction with monthly intravenous pulse cyclophosphamide, 750 mg/m2 body surface area, the dose adjusted to a maximum of 1 g/m2 based on nadir leucocyte counts (to be kept above 3000/cu mm) done on the tenth day following administration. Dosage was adjusted to renal function, with a 25% reduction in dose for an eGFR of <15 ml/min. All the patients received oral prednisone, (1 mg/kg/day) for six weeks and then gradually tapered, according to clinical improvement, by 10 mg/week to a maintenance dose of 5-7.5 mg/day. Eight patients received induction with mycophenolate mofetil (MMF), 500 mg three times daily, along with prednisone. All the patients received hydroxychloroquine, angiotensin-converting enzyme inhibitors, or angiotensin II receptor antagonists. Renal flares were treated with increasing oral prednisone or additional IV methylprednisolone pulses as required. Mesna was administered with cyclophosphamide and all the patients were given cotrimoxazole prophylaxis for the first 6 months.
36 of the 77 female patients were sexually active and were advised barrier contraception. They were counseled regarding the risks inherent to lupus in pregnancy and all of them agreed to defer pregnancy till disease quiescence.
The primary outcome measure was complete response (CR). This was defined, according to the EULAR consensus statement,[15] as inactive urinary sediment, a decrease in proteinuria to ≤0.2 g per day and normal or stable renal function. A partial response (PR) was defined as inactive urinary sediment, proteinuria ≤0.5 g per day, and normal or stable (if previously abnormal) GFR. Treatment failure was defined as any of the following – proteinuria of more than 3gm/day, a rise in creatinine of >0.6 mg/dl above the baseline, estimated GFR dropping to below 15% of the baseline value, or discontinuation of treatment due to side effects.[1516] Renal relapses were considered to be present if any of the following occurred (1) increase of proteinuria by 0.5 g/day to a value more than 1g/day in a patient previously in PR or CR (2) recurrence of active sediment (3) a decrease in estimated GFR by 30 ml/min. For those patients who had a proteinuria of >0.5 gm and <3 gm/day, and did not fit into either definitions of failure or remission, we defined them as with continuing disease activity.
Patients attended the out-patient department every two weeks for the first 6 months, then monthly for another 3 months and 2-monthly till the end of the study period. At each visit, blood pressure, SLEDAI scores, and occurrence of adverse events were noted. Laboratory tests done were 24-h protein excretion, C3, lipid profile, anti-dsDNA at baseline and at 6-monthly intervals. A complete hemogram, fasting sugar, liver function tests, urea, and creatinine were carried out at monthly intervals for the first 6 months and then with every visit. Estimated GFR (eGFR) was calculated from the MDRD equation (using three variables) for patients with age >16 years and the Schwartz equation[17] for patients at and below 16 years of age.
Secondary end points included partial response, any response (complete or partial), eGFRs, and proteinuria at 6 months and 1 year, adverse effects, renal relapses, treatment failures, progression to ESRD, or death.
Descriptive statistics are reported as frequency and percentage for categorical variables and as mean and standard deviation for continuous variables. One-way ANOVA was carried out to detect the differences, if any, in the baseline clinical and laboratory (continuous) variables among patients with partial, complete, or no responses at 6 months. Similarly, Chi square test was carried out to detect differences in categorical variables in the same groups. Pearson's and point bi-serial correlations were carried out to find out the relationship between eGFR, proteinuria, and response at 6 months and baseline, clinical, and laboratory values. Stepwise multiple regression analysis was carried out to detect predictors of outcome at 6 months in terms of proteinuria and eGFR. Logistic regression analysis was carried out to determine predictors of complete, partial and no remission.
Results
Baseline clinical and laboratory characteristics of patients are given in Tables 1 and 2. Mean follow-up of these patients was 13 months. Arthritis (79%), rash (64%), and fever (66%) were the most common manifestations at onset. The mean duration of symptoms prior to diagnosis was 13.9 months.


At 6 months, 18/86 (20.9%) patients were in partial response, 20 (23.3%) patients in complete response, and 12 (14%) patients were in treatment failure. The remaining 41.8% patients had continued disease activity but had reached neither end point yet. Three patients went into ESRD within 6 months of the onset of the induction period. Average time to achieve complete remission was 4.5 (±1.9) months and to achieve partial remission was 3.9 (±2.2 months). The time to achieve response did not correlate with the renal function and maintenance of response at 1 year. There were no differences in outcomes detectable in the three treatment groups at 1 year.
Forty-one of 86 patients (47.7%) had a low C3 at onset of which nine patients had a normalization of C3 levels at 1 month and 62 (72%) had a normal C3 level at 6 months. However, the C3 levels did not correlate with remission, and neither did normalization of C3 levels predict renal response.
Fifty-two of 86 patients presented with a baseline creatinine of <1.4 mg/dl, 14 (26.9%) of them achieved a complete response, and 13 patients (25%) a partial response at 6 months. Sixteen patients presented with a baseline creatinine of ≥2.5 mg/dl, of them four patients (25%) achieved a partial response, and one patient (6%) a complete response at 6 months. The presenting creatinine showed a trend toward a correlation with the response but did not reach statistical significance (P = 0.77).
Nine patients (10.5%) presented with an eGFR between 15-30 ml/min, and 7 (8%) patients presented with an eGFR of <15 ml/min. Five of them required dialysis at presentation. At 6 months, one patient among those presenting with an eGFR of <15 ml/min, achieved partial remission and three patients among them were in treatment failure; two developed end-stage renal disease (ESRD) in spite of 6 months of treatment with cyclophosphamide.
On one-way ANOVA [Table 3], there were significant differences in the baseline chronicity indices and the duration of symptoms prior to therapy, among patients with no, partial, or complete response. Patients with complete responses had a lower chronicity score and a shorter delay in treatment compared to patients with no response (P < 0.05). The baseline proteinuria correlated with the eGFR at 6 months (P = 0.009) and showed a trend with respect to attainment of complete or partial response (P = 0.091). There was a trend toward higher baseline hemoglobin levels in patients of complete response (P = 0.053). Presence of hypertension and leukopenia at the baseline was less likely in patients of complete or partial responses (P = 0.036 and 0.027 respectively). There was a trend toward the presence of nephrotic syndrome in non-responders (P = 0.077). There were no differences in the age at diagnosis, eGFR, gender, clinical features (arthritis, rash, oral ulcers, CNS disease, serositis, photosensitivity), C3 levels, serum albumin, biopsy class, or therapy in patients with no, partial, or complete remission. The class of biopsy (class III or IV) did not correlate with the response. The baseline and subsequent SLEDAI scores (done at monthly intervals) did not correlate with the response.
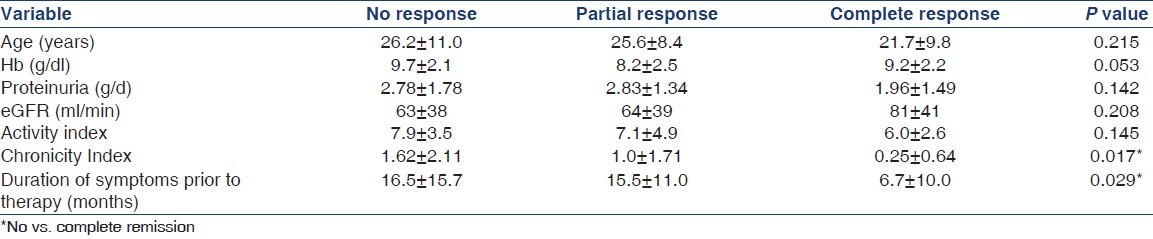
There was a significant correlation between eGFR at 6 months and age at diagnosis, hypertension, proteinuria, baseline renal function, percentage of glomeruli in the biopsy specimen containing the crescents, activity, and chronicity indices [Table 3]. Proteinuria at 6 months correlated with the chronicity index and hypertension. Response at 6 months correlated with age at diagnosis, hypertension, activity, chronicity indices, and duration of symptoms [Table 4]. The specific treatment given with mycophenolate or cyclophosphamide did not correlate with any of the outcome variables.

Stepwise multiple linear regressions were carried out with the aim to detect the predictors of eGFR at 6 months. In the final model, eGFR at baseline, chronicity index, and age at diagnosis were predictive of eGFR at 6 months, with an R2 of 0.521. Logistic regression analysis at 6 months, for any response (partial or complete), detected the absence of hypertension (Beta = 0.940, standard error = 0.465, P = 0.044) and chronicity index (beta = –0.330, standard error = 0.163, P = 0.043) as predictors. R2 (Nagelkerke) was 0.166.
There were no renal flares in the first 6 months of follow-up. Among the patients followed-up beyond 6 months, renal flares occurred in 2/42 patients (4.8%) in the 1st year and both were characterized by an increase in proteinuria but no active sediment. The time to flare was nine months in one patient and 11.5 months in the other. They responded to treatment, and went into remission by the 2nd year. Two of 26 patients (7.7%) had flares in the 2nd year, at 13 and 18 months respectively; these were also characterized only by the presence of proteinuria.
CNS involvement was present in 11/86 (12.8%) patients. Leucopenia was present in four patients, thrombocytopenia in eight patients (no overlap). Two or more organ-involvement was present in 17 (19.7%) patients. Presence of other organ involvement did not influence the renal response.
Twenty-two patients (25.6%) had mild infections within the first 6 months of therapy; 16 (18.6%) patients had severe infections requiring admission. One patient (male) developed disseminated tuberculosis; the majority of the rest were pneumonias. Incidence of severe infections decreased over the next 2 years, with two patients (4.9%) at 1 year and none at 2 years. Mild infections continued to occur; 13/42 patients (30%) had the infections in the 1st year and 13/26 patients in the 2nd year (50%). Nine patients (10.5%) developed amenorrhea, although, it subsequently improved in four of them with continuation of pulse cyclophosphamide – suggesting that disease activity may be a cause for amenorrhea in them. Three patients developed amenorrhea in the 1st year and none in the 2nd year. One patient developed a vertebral fracture after 15 months on corticosteroids. There were no deaths during this follow-up period.
Discussion
There has been a considerable improvement in the survival of patients with lupus nephritis, which has been attributed to an increased awareness, earlier referral to nephrologists, effectiveness of newer induction regimens, and an overall improvement in medical care. Various trials and studies comparing the outcomes in lupus nephritis have been confounded by the differences in histology (classes III, IV and V lesions), different treatment regimens, and different criteria for remission, relapse, and flare. The American College of Rheumatology (ACR) criteria for remission are a composite of estimated GFR of >90 ml/min, urinary protein to creatinine ratio <0.2 mg/mg, and inactive urinary sediment.[18] Inducing complete remission (including a normal renal function) is extremely difficult to achieve, especially in patients with higher chronicity indices. The EULAR response criteria, which have been adopted for this study, specify that a normal or stable GFR is acceptable for both complete and partial response.[15] Patients with refractory disease have been defined by the NIH as those who show no response to treatment and those in whom proteinuria does not decrease to less than half of pretreatment value or to <3 g per day and who have persistent active urinary casts or deterioration in serum creatinine level.[19]
Compared to the Caucasian[20] and African American patients,[21] the patients in the present study had a higher creatinine level but a lower degree of proteinuria at baseline. They also had a younger age at onset and lower activity, but higher chronicity indices on renal biopsy.[20]
The rates of response, in our patients, have been 21% and 23% at 6 months for CR and PR respectively. At 6 months, 53% patients in the cyclophosphamide arm of the ALMS study[22] reached the primary efficacy end point. This was defined as a decrease in urine protein/creatinine ratio (P/Cr), calculated from a 24-h urine collection, to <3 in patients with baseline nephrotic range P/Cr (≥3), or by ≥50% in patients with subnephrotic baseline P/Cr (<3).[23] A greater percentage of patients reached a response (partial or complete) at the end of the 1st year on follow-up (64%). In comparison, long-term follow-up of Chinese patients revealed a complete or at least partial response rate of 55% and 82%.[24] The figures for European Caucasian population were 62 and 88% in one study.[25] Median time to remission is usually longer than 6 months.[1026]
Our patients had an average delay of 14 months from the onset of the disease to the initiation of therapy, and this delay was more in patients who achieved no response versus those who achieved complete response – this is a known cause for refractory disease.[12]
A decrease in proteinuria is often a marker for better renal outcomes. In our patients, response was associated with a non-significant trend toward lesser degrees of proteinuria (P = 0.055). In the long-term outcomes of the Euro–Lupus cohort, it was demonstrated by multivariate analysis that early response to therapy, by 6 months, (defined as a decline in creatinine and a decrease in proteinuria at 6 months to less than 1 gm/day) was the best predictor of long-term outcomes.[27]
A younger age at diagnosis is considered to be a poor prognostic marker. In our study, the age of patients at diagnosis ranged from 5 to 48 years. Ten patients were less than 12 years of age at the time of diagnosis. Over this age range, the age at diagnosis (as well as the present age) was negatively correlated with response and eGFR at 6 months. This may have been due to a longer latency for treatment in those of older age. Other markers of poor prognosis – hypertension, renal impairment at baseline, presence of crescents, and a higher chronicity index at baseline were also corroborated in our study.[828–31]
The reported rates of relapse vary from 25% at 5 years, to 46% at 10 years.[3233] The duration of follow-up was too short to provide meaningful rates of relapse, but two patients had relapsed in the 1st and 2nd years. 14% of the patients experienced treatment failure after the first 6 months. This is similar to the rate of treatment failure in the Euro Lupus trial (16% in the low-dose cyclophosphamide arm; 20% in the high-dose arm).[20]
A study from south India revealed that remission rates in a cohort of predominantly Class IV lupus patients were 82.05% and average time to remission was 15 months. In this study, early diagnosis, a higher creatinine at presentation, and ACEi/ARB use predicted remission.[34] Another study by the same authors highlighted that cyclophosphamide therapy in a group of 39 patients (75% Class IV) achieved complete remission in 44.8% of patients after a mean follow-up of 15.8 months.[35] In another study which looked at long-term survival in lupus nephritis, risk factors for poor outcome were low C3, hematuria, hypertension, creatinine, lack of remission, and occurrence of a major infection.[36] Another report highlighted that pediatric lupus had slightly better outcomes – 84.6% were in complete or partial remission at 1 year.[37] In the pediatric population, a study from eastern India revealed that overall female to male ratio was 3.8:1. Renal manifestations were present in 54% of the patients.[38] Another report from north India evaluated 25 children with lupus nephritis. Cyclophosphamide pulses were given in eight patients, four of whom became asymptomatic after 4-24 months of therapy.[39] Diffuse proliferative glomerulonephritis (WHO class IV) is the predominant histological presentation in children and is more common in boys than girls.[40] In the adult patients, men had more severe renal impairment (60% vs. 37.5%), with higher levels of mean serum creatinine (2.67 vs. 1.62 mg/dl), and blood urea (63.25 vs. 48 mg/dL) compared to women.[41] The phenotypic expression of renal disease is severe in Indians. A study on serum levels of interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin-4 (IL-4), and interlekin-10 (IL-10) were carried out in Indian patients. TNF-α and IFN-γ were positively correlated and IL-10 and IL-4 were negatively correlated with SLEDAI scores. The authors concluded that TNF-α contributed significantly to pathological manifestations of SLE in this region.[42]
In the present study, we evaluated a population of patients with SLE class IV nephritis. We assessed response to therapy and its predictors in the short term. At baseline, age proved to be negatively correlated to outcomes. Age at diagnosis, the chronicity index, and the eGFR at baseline provided 52% of the variance to predict the eGFR at 6 months. Other factors for poor prognosis were hypertension, proteinuria, eGFR at baseline, percentage of glomeruli in the biopsy specimen containing crescents, and activity and chronicity indices.
Source of Support: Nil
Conflict of Interest: None declared.
References
- The natural history of the renal manifestations of systemic lupus erythematosus. J Lab Clin Med. 1964;63:537-50.
- [Google Scholar]
- Long-term survival in systemic lupus erythematosus. Patient characteristics associated with poorer outcomes. Arthritis Rheum. 1995;38:274-83.
- [Google Scholar]
- Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int. 2006;69:1846-51.
- [Google Scholar]
- Demographic differences in the development of lupus nephritis: A retrospective analysis. Am J Med. 2002;112:726-9.
- [Google Scholar]
- Long-term outcome of patients with diffuse proliferative lupus nephritis treated with prednisolone and oral cyclophosphamide followed by azathioprine. Lupus. 2005;14:265-72.
- [Google Scholar]
- Survival analysis and prognostic indicators of systemic lupus erythematosus in Pakistani patients. Lupus. 2009;18:848-55.
- [Google Scholar]
- High-risk features of lupus nephritis: Importance of race and clinical and histological factors in 166 patients. Nephrol Dial Transplant. 1995;10:1620-8.
- [Google Scholar]
- Factors predictive of outcome in severe lupus nephritis. Lupus nephritis collaborative study group. Am J Kidney Dis. 2000;35:904-14.
- [Google Scholar]
- Baseline characteristics of a multiethnic lupus cohort: Profile. Lupus. 2002;11:95-101.
- [Google Scholar]
- Remission, relapse, and re-remission of proliferative lupus nephritis treated with cyclophosphamide. Kidney Int. 2000;57:258-64.
- [Google Scholar]
- Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
- [Google Scholar]
- The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241-50.
- [Google Scholar]
- European consensus statement on the terminology used in the management of lupus glomerulonephritis. Lupus. 2009;18:257-63.
- [Google Scholar]
- Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong Guangzhou Nephrology Study Group. N Engl J Med. 2000;343:1156-62.
- [Google Scholar]
- Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832-43.
- [Google Scholar]
- The American college of rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum. 2006;54:421-32.
- [Google Scholar]
- Outcome criteria for lupus nephritis trials: A critical overview. Lupus. 1998;7:622-9.
- [Google Scholar]
- Immunosuppressive therapy in lupus nephritis: The euro-lupus nephritis trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121-31.
- [Google Scholar]
- Influence of race/ethnicity on response to lupus nephritis treatment: The ALMS study. Rheumatology (Oxford). 2010;49:128-40.
- [Google Scholar]
- Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103-12.
- [Google Scholar]
- Nonrenal disease activity following mycophenolate mofetil or intravenous cyclophosphamide as induction treatment for lupus nephritis: Findings in a multicenter, prospective, randomized, open-label, parallel-group clinical trial. Arthritis Rheum. 2010;62:211-21.
- [Google Scholar]
- Predictors and outcome of renal flares after successful cyclophosphamide treatment for diffuse proliferative lupus glomerulonephritis. Arthritis Rheum. 2004;50:2559-68.
- [Google Scholar]
- The long-term outcome of 93 patients with proliferative lupus nephritis. Nephrol Dial Transplant. 2007;22:2531-9.
- [Google Scholar]
- Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med. 1996;125:549-57.
- [Google Scholar]
- Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term follow up of patients in the Euro-Lupus Nephritis Trial. Arthritis Rheum. 2004;50:3934-40.
- [Google Scholar]
- The clinical and renal biopsy predictors of long-term outcome in lupus nephritis: A study of 87 patients and review of the literature. Q J Med. 1989;72:779-833.
- [Google Scholar]
- Lupus nephritis: prognostic factors and probability of maintaining life-supporting renal function 10 years after the diagnosis. Gruppo Italiano per lo Studio della Nefrite Lupica (GISNEL) Am J Kidney Dis. 1992;19:473-9.
- [Google Scholar]
- Predicting renal outcomes in severe lupus nephritis: Contributions of clinical and histologic data. Kidney Int. 1994;45:544-50.
- [Google Scholar]
- Prognostic factors of diffuse proliferative lupus nephritis. Clin Nephrol. 1999;52:139-47.
- [Google Scholar]
- “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int. 1996;50:2047-53.
- [Google Scholar]
- Cumulative rate of relapse of lupus nephritis after successful treatment with cyclophosphamide. Arthritis Rheum. 1996;39:2028-34.
- [Google Scholar]
- The outcome of proliferative lupus nephritis with pulse cyclophosphamide therapy. Indian J Nephrol. 2011;21:160-5.
- [Google Scholar]
- Pulse cyclophospamide in severe lupus nephritis: Southern Indian experience. Saudi J Kidney Dis Transpl. 2010;21:372-8.
- [Google Scholar]
- Long-term outcome of lupus nephritis in Asian Indians. Arthritis Care Res (Hoboken). 2012;64:713-20.
- [Google Scholar]
- Childhood lupus nephritis: 12 years experience from North India. Rheumatol Int. 2006;26:604-7.
- [Google Scholar]
- Sex-based differences in lupus nephritis: A study of 235 Indian patients. J Nephrol. 2008;21:570-5.
- [Google Scholar]
- Cytokine imbalance in systemic lupus erythematosus: A study on northern Indian subjects. Lupus. 2012;21:596-603.
- [Google Scholar]







