Translate this page into:
Clinico-microbiological Profile and Outcomes of Asymptomatic Bacteriuria in Pregnancy
Corresponding author: Dr. Saritha Suryadevara, Department of Nephrology, M S Ramaiah Medical College, Rajiv Gandhi University of Health Sciences, Bengaluru - 560 054, Karnataka, India. E-mail: suryadevara477@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Eshwarappa M, Rao MY, Gurudev KC, Gireesh MS, Swaroop A, Suryadevara S. Clinico-microbiological Profile and Outcomes of Asymptomatic Bacteriuria in Pregnancy. Indian J Nephrol. 2024;34:134–8. doi: 10.4103/ijn.ijn_305_21
Abstract
Background:
Asymptomatic bacteriuria (ASB) during pregnancy can lead to symptomatic urinary tract infection (UTI), with increased fetal and maternal morbidity and mortality. We evaluated the incidence, clinical and microbiological profile, and outcome of ASB in pregnant women attending our antenatal clinic.
Materials and Methods:
This prospective study was conducted on 3769 pregnant women in a routine antenatal clinic at a tertiary care center. Participants were divided into two groups, ASB and non-bacteriuria. Data were collected in a standard proforma and analyzed using the software Statistical Package for the Social Sciences (SPSS) v. 20.
Results:
The incidence of ASB was 3.29% (124/3769). Majority of the women were in the age group of 21–30 years (78.76%, n = 89). Escherichia coli (61.06%) was the most common organism isolated. Maternal anemia (30.08% and 2.93% in the ASB and non-bacteriuria groups, respectively), low birth weight (LBW; 42.5% and 27.98% in the ASB and non-bacteriuria groups, respectively), intrauterine death (4.4% and 1.4% in the ASB and non-bacteriuria groups, respectively), and preterm delivery (37.2% and 22.31% in the ASB and non-bacteriuria groups, respectively) were were associated with ASB (P = 0.001).
Conclusion:
ASB was associated with maternal anemia, preterm delivery, intrauterine death, and LBW. Early detection and treatment of ASB may result in favorable maternal outcome.
Keywords
Bacteriuria
drug resistance
Escherichia coli
pregnant women
urinary tract infections
Introduction
Urinary tract infection (UTI) during pregnancy has an adverse effect on the mother, fetus, and the newborn. Pregnant women experience morphological and physiological changes in the genitourinary tract, which predispose them to UTIs.1 UTIs during pregnancy are of two types: symptomatic and asymptomatic urinary infections.2 Asymptomatic bacteriuria (ASB)1 can develop in 2% to 10% of pregnant women.3 ASB can lead to acute pyelonephritis in 20%–50% of cases with other adverse obstetric outcomes such as prematurity, hypertension complicating pregnancy, anemia, low birth weight (LBW), and higher fetal mortality.4,5 Escherichia coli accounts for 80%–90% of infections and is the most frequently isolated organism.6
The US Preventive Services Task Force (USPSTF) and the American Academy of Family Physicians (AAFP) strongly recommend for ASB in pregnant women at 12–16 weeks gestation.7,8 Similarly, the American College of Obstetricians and Gynecologists (ACOG) recommends screening of all pregnant women for ASB at the first prenatal visit and a repeat urine culture during the third trimester.9
Age, level of sexual activity, genitourinary abnormalities, personal hygiene, and socioeconomic status affect ASB prevalence. Advancing age is an important risk factor, with a prevalence as low as ∼1% among schoolgirls to ∼20% among healthy women ≥80 years of age living in the community. Amongst medical factors, abnormalities of urinary tract or stones, immunosuppression, sexual activity, anemia, diabetes mellitus, and previous history of UTI increase the risk.10
The neonatal and maternal complications of ASB during pregnancy can be devastating. Nearly 30% develop symptomatic cystitis and up to 50% develop pyelonephritis.11 Maternal complications include anemia, premature rupture of membranes (PROM), recurrent UTI, preterm labor, cystitis, and acute pyelonephritis. Fetal complications commonly reported are LBW, intrauterine growth restriction, and even intrauterine death (IUD).
We evaluated the incidence and the clinical and microbiological profiles and outcomes of ASB in pregnant women attending our antenatal clinic.
Materials and Methods
This is a prospective study conducted at an antenatal clinic in M S Ramaiah group of hospitals attached to M S Ramaiah Medical College, Bangalore over a 18 month period (February 2016 to July 2017). All pregnant women attending the antenatal clinic were included and divided into two groups, that is, ASB group and non-bacteriuria group. Detailed clinical and laboratory data were collected. All study participants were subjected to routine investigations, which included complete blood counts and routine urine examination. Those study participants who had >5 pus cells/high-power field (HPF) in spun urine on microscopic examination were further evaluated with urine culture(s). If the first sample of urine culture was positive for bacterial growth, then repeat culture was done. Those with positive second culture for the same organism were treated with antibiotics for 3–7 days based on the standard treatment guidelines practiced at our institution. We did not repeat a urine culture post antibiotic treatment due to financial burden on the patient, but a urine examination for any pus cells was routinely done. Cases with pyuria (pus cells >5/HPF) were also included in the ASB group. The following groups of patients were excluded from the study: those patients with an indwelling catheter at the time of sampling, those who were lost to follow-up, those who refused consent, those with a history of symptomatic and recurrent UTIs in current pregnancy, and patients with structural and functional abnormalities of the urinary tract.
Calibrated loop direct streak method was used for bacterial culture. Colonies of potential pathogens were looked for and carefully counted on both plates. After the plate count was done, the organism was identified by conventional methods and antibiotic susceptibility testing was performed by disk diffusion technique.
All patients were followed up until delivery. Patients who did not turn up for further follow-up and for delivery were contacted on their mobile phones and the required information was collected. Compiled data was analyzed to study the clinical profile as well as maternal and fetal outcome. Age, gestational period, parity, and the mode of delivery were examined. Maternal adverse outcomes like hypertension complicating pregnancy, anemia, PROM, preterm PROM (PPROM), and preterm delivery were studied. Fetal outcomes like LBW, intrauterine growth retardation (IUGR), and IUD were also analyzed. Incidence of gestational diabetes and hypothyroidism was also noted.
Statistical analysis
The data was recorded using MS Excel, and analysis was carried out using the statistical software Statistical Package for the Social Sciences (SPSS) v. 20. Mean ± standard deviation, Chi-square test for linear trend, and relative risk were used to find the difference between the two groups. P ≤ 0.05 was considered statistically significant.
Results
This study enrolled 3769 pregnant women, out of whom 124 were found to have ASB [Figure 1]. By the end of the study period, only 113 patients with ASB could be analyzed, as 11 patients were not accessible for data collection. The remaining 3645 pregnant women were included in the non-bacteriuria group, of whom 32 were excluded from the analysis due to inadequate data, giving us a final figure of 3613. ASB was seen in 3.29% of pregnant women.
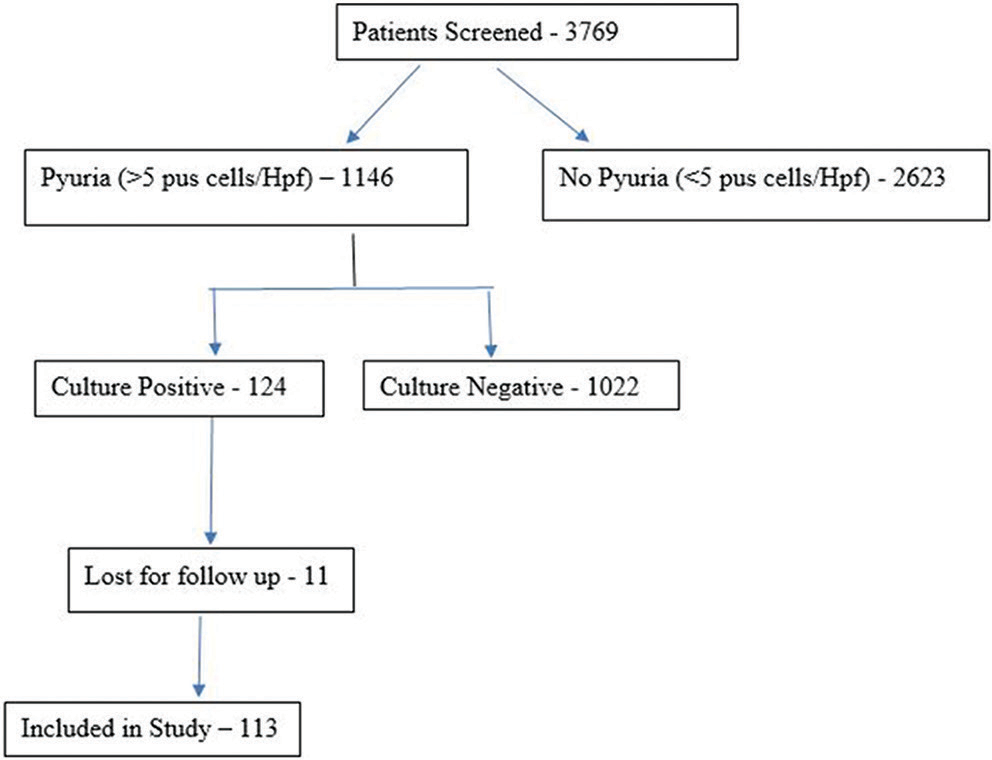
- Algorithm of the study.
Table 1 shows that most of the pregnant women in the ASB (38.05%) and non-bacteriuria (38.06%) groups were aged 21–25 years. Also, the mean age (P = 0.390) was found to be 25.88 and 26.47 years in the ASB and non-bacteriuria groups, respectively.
| Characteristic | ASB group n (%) | Non-ASB group n (%) | |
|---|---|---|---|
| Age, years | |||
| 16–20 | 13 (11.50) | 252 (6.97) | |
| 21–25 | 43 (38.05) | 1375 (38.06) | |
| 26–30 | 46 (40.71) | 1363 (37.73) | |
| 31–35 | 7 (6.20) | 498 (13.78) | |
| >35 | 4 (3.54) | 125 (3.46) | |
| Total | 113 (100) | 3613 (100) | |
| Maternal comorbidities | |||
| Anemia | 34 (30.08) | 106 (2.93) | <0.001* |
| Chronic hypertension | 3 (2.65) | 0 (0) | 0.870 |
| Gestational hypertension | 6 (5.31) | 188 (5.20) | 0.960 |
| Pre-eclampsia | 17 (15.04) | 379 (10.49) | 0.122 |
| Eclampsia | 3 (2.65) | 34 (0.94) | 0.07 |
| PROM | 4 (3.54) | 179 (4.95) | 0.493 |
| PPROM | 7 (6.19) | 149 (4.12) | 0.279 |
| Hypothyroidism | 10 (8.85) | 430 (10.41) | 0.322 |
| GDM | 6 (5.31) | 161 (6.03) | 0.573 |
ASB=asymptomatic bacteriuria, GDM=gestational diabetes mellitus, non-ASB=non-asymptomatic bacteriuria, PPROM=preterm premature rupture of membranes, PROM=premature rupture of membranes. *Significant
We compared the general maternal characteristics and comorbidities and analyzed the odds ratio and fetal outcomes in both ASB and non-bacteriuria groups [Table 1]. ASB had a statistically significant relationship with maternal anemia (P < 0.001), chronic hypertension (P < 0.001), preterm delivery (P = 0.001), LBW (P = 0.001), and IUD (P = 0.022).
Incidence of anemia was higher among pregnant women with ASB than those in the non-bacteriuria group [34 (30.08%) vs 106 (2.93%)]. All fetal outcomes were poorer in the ASB group, with higher incidence of preterm deliveries: 42 (37.2%) vs 806 (22.31%) and LBW: 48 (42.5%) vs 1011 (27.98%).
Table 2 details the other comorbidities during the second and third trimesters. Chronic hypertension and anemia were detected in early pregnancy. The incidence of ASB was highest in the third trimester, followed by the second and first trimesters, with 77 (68.14%), 26 (23%), and 10 (8.85%) cases, respectively [Table 3]. LBW was noticed in 30 (38.96%) in their third trimester, 13 (50%) in their second trimester, and three (30%) women in first trimester. Incidence of ASB and trimester of pregnancy did not affect the incidence of LBW (P = 0.748).
| Parameter studied | ASB group, n (%) | Non-ASB group, n (%) | P |
|---|---|---|---|
| Maternal characteristics | |||
| Primigravida | 58 (51.33) | 1521 (42.09) | 0.051 |
| Gravida ≥2 | 55 (48.67) | 2092 (57.91) | 0.369 |
| Abortions | 27 (23.89) | 969 (26.8) | 0.488 |
| Vaginal delivery | 69 (61.06) | 1991 (55.11) | 0.144 |
| Elective Caesarian section | 6 (5.31) | 264 (7.3) | 0.420 |
| Emergency Caesarian section | 38 (33.63) | 1361 (37.66) | 0.382 |
| Twin pregnancies | 5 (4.42) | 97 (2.68) | 0.264 |
| Fetal outcomes | |||
| Preterm delivery | 42 (37.2) | 806 (22.31) | 0.001* |
| Low birth weight | 48 (42.5) | 1011 (27.98) | 0.001* |
| IUD | 5 (4.4) | 51 (1.4) | 0.022* |
ASB=asymptomatic bacteriuria, IUD=intrauterine death, non-ASB=non-asymptomatic bacteriuria, * - Significant P
| Trimester | ASB detected in each trimester n (%) | LBW observed in each trimester n (%) | Percentage of LBW out of total ASB cases studied | P |
|---|---|---|---|---|
| First trimester | 10 (8.85) | 3 (6.25) | 30 | 0.748 |
| Second trimester | 26 (23) | 13 (27.08) | 50 | |
| Third trimester | 77 (68.14) | 30 (62.5) | 38.96 |
ASB=asymptomatic bacteriuria, LBW=low birth weight
E. coli was the predominantly isolated organism (61.06%) [Figure 2]. Extended-spectrum beta-lactamase E. coli accounted for 45.13% of the isolates, followed by Enterococcus species (15.04%), Klebsiella species (12.38%), Citrobacter species (7.07%), Proteus (3.53%), and Staphylococcus species (0.88%).

- Bacterial isolates among culture-positive cases.
The pattern of antibiotic susceptibility to isolated strains is shown in Figure 3. The gram-positive organisms were all sensitive to vancomycin, erythromycin, clindamycin, linezolid, cotrimoxazole, teicoplanin, cloxacillin, and doxycycline. The gram-negative organisms isolated displayed variable sensitivity and resistance patterns, with reasonably good susceptibility to amikacin (86.31%), imipenem (82.3%), meropenem (68.75%), nitrofurantoin (75.22%) and gentamicin (59.38%). Sensitivity to other antibiotics varied from 33.33% to 47.92%. In our institute, nitrofurantoin was the most prescribed antibiotic, used in 75% of patient population, followed by oral cephalosporins, meropenem, and imipenem. For the gram-positive group, the most common antibiotic used was linezolid.
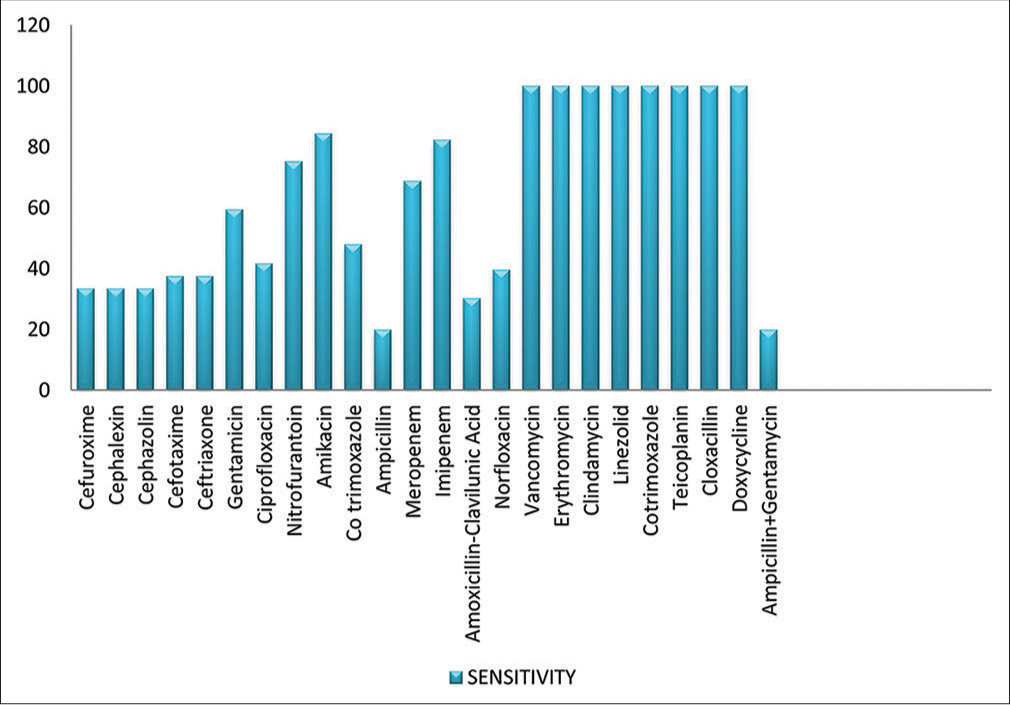
- Culture and sensitivity patterns among culture-positive cases of pregnant women with ASB. ASB = asymptomatic bacteriuria.
Discussion
ASB in pregnancy is an important cause of maternal morbidity and adverse fetal outcome. The incidence of ASB in our study was 3.29%. The global incidence of ASB during pregnancy is reported to range from 2% to 10%.12 Jennifer et al.13 reported an incidence of 3.6% in their study. Some of the Indian studies such as Sujatha et al.14 and Radha et al.15 have reported an incidence of 7.3% and 8.25%, respectively. The incidence of ASB in our study is in concordance with similar studies across the world, but slightly lower than what has been reported in Indian studies.14,15 The difference may be due to the differences in socioeconomic conditions of the populations studied.
Majority of the pregnant women (78.76%) enrolled in this study were aged 21–30 years, similar to that reported by Sujatha et al.14 This is the age at which many women in India get married and have children, exposing them to higher risk for ASB.
We analyzed the mode of delivery in both the ASB and non-bacteriuria groups of pregnant women and whether ASB during pregnancy increased the incidence of cesarean section, either elective or emergency. However, no statistically significant differences were noticed in the modes of delivery in either group, in agreement with the findings reported by Radha et al.15
The incidence of LBW and anemia in the ASB group was higher than in the non-bacteriuria group. The incidence of anemia was higher in second and third trimesters of pregnancy in the ASB group. Preterm birth was more common in pregnant women with ASB than those in the non-bacteriuria group, with a statistically significant difference. Radha et al.15 and Verma et al.16 showed slightly lower figures for LBW, anemia, and preterm birth weight in pregnant women with ASB in their studies. The present study found a relatively higher incidence of anemia and preterm births in the ASB group than what has been previously reported by others. The reason for such a difference could be that our patients presented with ASB in their third and second trimesters of pregnancy.
The incidence of ASB in our study was highest in the third trimester followed by the second and first trimesters, similar to what was reported by Gayathree et al.17 and Ramalingam et al.18 Subgroup analysis according to the trimester of pregnancy showed that majority of the comorbidities like anemia, chronic hypertension, gestational hypertension, pre-eclampsia, eclampsia, PROM, and PPROM occurred late during the third trimester.
The most common bacterial isolate in our study was E. coli, followed by Enterococcus and Klebsiella species. Sujatha et al.14 showed that E. coli was the most common isolate (77%), followed by Klebsiella and Enterococcus species. Mukherjee et al.19 showed that the incidence of E. coli was 57.14%, followed by Klebsiella pneumoniae and Staphylococcus species.
We found that nitrofurantoin was the commonly prescribed antibiotic, followed by amikacin, meropenem, and imipenem. Sensitivity to nitrofurantoin was reported in nearly 75.22% of isolates. We compared our sensitivity patterns with those reported by Prasanna et al.20 and Valentina et al.21 The sensitivity pattern of nitrofurantoin and amikacin in our study was similar to that reported by Prasanna et al.,20 where they also reported higher sensitivity to ceftriaxone, meropenem, and norfloxacin. Valentina et al.21 reported much lower sensitivity patterns to the antimicrobials in comparison to the present study. This difference may reflect the regional variation in the sensitivity patterns of the organisms and institutional antibiotic protocols. Judicious use of antibiotics has now become the need of the hour to prevent emergence of resistant strains.
We did not find any correlation with earlier abortion and ASB in current pregnancy. This study does not show the effect of untreated ASB in pregnancy.
Conclusion
The incidence of ASB in our study was 3.29% (124/3769). Maternal anemia, preterm delivery, IUD, and LBW were the most common outcomes noted in the ASB group. We recommend mandatory screening for ASB during early pregnancy for better maternal and fetal outcomes.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Infectious Diseases Society of America Guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-54.
- [CrossRef] [PubMed] [Google Scholar]
- Asymptomatic and symptomatic urinary tract infections: Magnitude, special settings and diagnostic testing. JCEHP. 2012;14:88-94.
- [Google Scholar]
- Asymptomatic bacteriuria and antibacterial susceptibility patterns in an obstetric population. ISRN Obstet Gynecol. 2011;2011:721872.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of rapid urine screening tests to detect asymptomatic bacteriuria in pregnancy. Jpn J Infec Dis. 2006;59:261-3.
- [CrossRef] [Google Scholar]
- Hygiene practices and sexual activity associated with urinary tract infection in pregnant women. East Mediterr Health J. 2009;15:104-10.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for asymptomatic bacteriuria in adults: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:1195-205.
- [CrossRef] [Google Scholar]
- Summary of recommendations for clinical preventive services. Revision 6.4 August 2007. Available from: https://www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/cps-recommendations.pdf
- [Google Scholar]
- Antimicrobial therapy for obstetric patients In: ACOG educational bulletin no. 245 (8-10). Washington, DC: American College of Obstetricians and Gynecologists; 1998.
- [Google Scholar]
- Other infectious conditions in pregnancy In: James DK, Steer PJ, Weiner CP, Govik B, eds. High Risk Pregnancy: Management Options (2nd ed). London: WB Saunders; 1999. p. :559-98.
- [Google Scholar]
- Pregnancy, pyelonephritis and prematurity. Clin Obstet Gynecol. 1970;13:239-54.
- [CrossRef] [PubMed] [Google Scholar]
- Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database Syst Rev 2011:CD000491.
- [CrossRef] [Google Scholar]
- Asymptomatic bacteriuria in pregnancy: Prevalence, risk factors and causative organisms. Sri Lankan Journal of infectious diseases. 2012;1(2):42-46.
- [CrossRef] [Google Scholar]
- Prevalence of Asymptomatic bacteriuria and its antibacterial susceptibility pattern among pregnant women attending the antenatal clinic at Kanpur, India. J Clin Diagn Res. 2014;8:1-3.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and outcome of asymptomatic bacteriuria in early pregnancy. Int J Reprod Contracept Obstet Gynecol. 2017;6:223-7.
- [CrossRef] [Google Scholar]
- Asymptomatic bacteriuria in pregnancy and its relation to perinatal outcome. Int J Reprod Contracept Obstet Gynecol. 2016;5:4390-6.
- [CrossRef] [Google Scholar]
- Screenning for asymptomatic bacteriuria in pregnancy: An evaluation of various screening tests in Hassan District Hospital, India. J Clin Diagn Res. 2010;4:2702-6.
- [Google Scholar]
- Prevalence of asymptomatic bacteriuria in antenatal women coming to NRIMC & GH. Bangladesh J Obstet Gynaecol. 2015;30:30-6.
- [CrossRef] [Google Scholar]
- A study on asymptomatic bacteriuria in pregnancy: Prevalence, etiology and comparison of screening methods. Int J Res Med Sci. 2014;2:1085-91.
- [CrossRef] [Google Scholar]
- Prevalence of asymptomatic bacteriuria in pregnant women, isolates and their culture sensitivity pattern. Int J Curr Microbiol App Sci. 2015;4:28-35.
- [Google Scholar]
- Pregnancy associated urinary tract infection: Prevalence and screening. Int J Curr Microbiol App Sci. 2016;5:452-60.
- [CrossRef] [Google Scholar]







