Translate this page into:
Cognitive Dysfunction Screening in Peritoneal Dialysis Patients: A Cross-Sectional Study
Corresponding author: Ana C. Brás, Department of Nephrology, Hospital Professor Doutor Fernando Fonseca, Lisboa, Portugal. E-mail: anacatarinabras@msn.com
-
Received: ,
Accepted: ,
How to cite this article: Brás AC, Marques J, Fernandes V, Ferreira AC. Cognitive Dysfunction Screening in Peritoneal Dialysis Patients: A Cross-Sectional Study. Indian J Nephrol. 2024;34:357-62. doi: 10.25259/ijn_378_23
Abstract
Background:
Mild cognitive impairment (MCI) in peritoneal dialysis (PD) patients has been described as a risk factor for worse outcomes such as peritonitis, technique failure, and mortality. In this study, we aimed to determine the prevalence of MCI in a population of PD patients and identify the possible risk factors associated with MCI.
Materials and Methods:
We performed an observational, cross-sectional study to evaluate cognitive function using the Montreal Cognitive Assessment (MOCA) test and the Mini Mental State Examination (MMSE) test in PD patients. Patients with diagnosis of dementia or severe neurologic impairment, active cancer, or infection were excluded.
Results:
We evaluated 66 patients (mean age 60 years); 53% were male. Prevalence of MCI assessed by MOCA test and MMSE test was 65% and 33%, respectively. Predictors of MCI with MOCA test were higher age (P = 0.0001), lower education level (P = 0.005), need of a helper (P = 0.009), and continuous ambulatory PD modality (P = 0.019). Higher Charlson comorbidity index (P = 0.002), coronary artery disease (P = 0.006), and peripheral artery disease (P = 0.033) were also associated with MCI. Lower Kt/V (P = 0.012) and lower levels of normalized protein catabolic rate (nPCR; P < 0.000) were related to MCI. MCI patients had more episodes of peritonitis (P = 0.047). Multivariable analysis showed that lower education, Kt/V, and nPCR were the most relevant factors connected to MCI (P = 0.029, P = 0.037, and P = 0.019, respectively).
Conclusion:
In our PD population, MCI was detected in more than half of the patients. Patients with MCI were older, had lower education level, more disease burden, and higher risk for developing peritonitis. Lower Kt/V and nPCR levels were associated with MCI.
Keywords
Cognitive dysfunction
Dialysis adequacy
Dialysis nutrition
Mild cognitive impairment
Peritoneal dialysis
Introduction
Mild cognitive impairment (MCI) in patients with end-stage chronic kidney disease (ESKD) on peritoneal dialysis (PD) negatively affects their treatment,1-4 increasing the risk of hospitalization, technique failure, peritonitis, and mortality.5-7 Although not fully understood, several mechanisms have been described to explain MCI in patients with renal disease, such as vascular dysfunction, neuroinflammation, oxidative stress, and uremia.8-12 The growing interest and need for development in this area has led to the creation of Cognitive decline in Nephro-Neurology: European Cooperative Target (CONNECT) working group.
The prevalence of MCI on PD ranges from 3% to 75%, with an average of 29%.2,11,13,14 While some authors defend a lower prevalence of MCI in PD patients compared to hemodialysis (HD) patients,15 others do not have the same opinion.16 As in nondialyzed patients, the prevalence of MCI increases with age,2,17,18 and other factors are associated with an increased risk of MCI in dialysis patients, such as female gender, low education, diabetes, and cardiovascular disease.3,7,19-21 Dialysis adequacy, such as urea clearance (measured by Kt/V), and nutritional parameters have also been linked to MCI.13,22
Currently, several tools are available for MCI screening. Montreal cognitive assessment (MOCA) and Mini mental state examination (MMSE) are two examples of simple and easy-to-apply tests, validated for assessment of global cognitive function.23 Recognition of MCI and associated risk factors in PD population is necessary to prevent and mitigate negative outcomes.
In this study, we aimed to 1) determine the prevalence of MCI in a population of PD patients, 2) identify the possible risk factors associated with MCI, and 3) find an association between MCI and catheter-related infection. A secondary aim was to study whether attending nephrologists could detect MCI in these patients.
Materials and Methods
Study design and subjects
An observational, cross-sectional study was approved by our local ethics committee. The study protocol was reviewed and approved from the Institutional Ethics Committee of Central Lisbon University Hospital Centre (CHULC) with the approval number 1254/2022, dated 08th November 2022. Informed consent was obtained from all participants.
Sixty-nine patients on PD were screened. The inclusion criteria were age ≥18 years, active follow-up in our PD program, and ability to complete the questionnaires. Exclusion criteria were previous diagnosis of dementia or severe neurologic impairment, active infection, active cancer, and refusal to participate in the study.
Sixty-six patients were enrolled in the study. Three were excluded (one with active cancer, one with dementia, and one with severe neurologic disease). All patients answered two tests that evaluated global cognitive function: MOCA and MMSE test. The cut-off point of MOCA score was <26; for subjects with less than 12 years of education, one point was added to the score. For MMSE, the cut-off points were ≤15 for illiterate subjects, ≤22 for subjects with 11 years of education, and ≤27 for patients with more than 11 years of education.
Two of the treating nephrologists provided a binary answer to the question whether their patient had MCI or not, before knowing the tests results.
Data collection and statistical analysis
Demographic and clinical data (such as age, gender, level of education, need of a helper, associated comorbidities, body mass index) were collected through clinical registries. Laboratory evaluation of hemoglobin, urea, creatinine, albumin, sodium, C-reactive protein, parathyroid hormone, bicarbonate, and vitamin D was performed on the same day the questionnaires were used.
Data regarding dialysis adequacy, such as residual renal function, urea and creatinine clearance, and nutrition parameters such as normalized protein catabolic rate (nPCR), were also obtained. These parameters were calculated from 24-h urine and dialysate effluent collections. Peritonitis and exit-site infections were recorded during a 6-month period.
Statistical data analysis was performed using Statistical Package for the Social Sciences (SPSS) software application. Data are shown as mean ± standard deviation (SD) for normally distributed variables and median (interquartile range [IQR]) otherwise. Binary variables were presented as frequencies (percentage). Comparisons between groups were performed with Mann–Whitney U test and Chi-square test. Multivariable analysis using logistic regression analysis was performed to identify the variables that better correlated with MCI. Statistical significance was defined as a P- value less than 0.05.
Results
Table 1 shows the demographic information and clinical features of the study population. Of the 66 patients included in the study, 53% were male. The mean age of patients was 59.7 ± 15.0 years. Arterial hypertension (83%), cardiovascular disease (38%), and diabetes (38%) were the most common comorbidities. Median Charlson comorbidity index (CCI) score was 5 (2.75–7.00).
| Variable | Total n = 66 | Control n = 23 | MCI by MOCA test n = 43 | P |
|---|---|---|---|---|
| Age, mean ± SD | 59.7 ± 15.0 | 52.0 ± 13.1 | 64.0 ± 14.0 | 0.001 |
| Male gender, n (%) | 35 (53.0) | 11 (47.8) | 24 (55.8) | 0.536 |
| Body mass index (kg/m2), mean ± SD | 26.2 ± 4.2 | 24.3 ± 3.3 | 27.1 ± 4.1 | 0.014 |
| Education, n (%) | 0.005 | |||
| ≤4 years | 14 (21.2) | 0 (0) | 14 (32.6) | |
| 5–9 years | 14 (21.2) | 3 (13.0) | 11 (25.6) | |
| 10–11 years | 20 (30.3) | 12 (52.2) | 8 (18.6) | |
| ≥12 years | 18 (27.3) | 8 (34.8) | 10 (23.3) | |
| Professionally active, n (%) | 25 (37.9) | 15 (65.2) | 10 (23.3) | 0.001 |
| Live alone, n (%) | 10 (15.2) | 3 (13.0) | 7 (16.3) | 0.727 |
| Arterial hypertension, n (%) | 55 (83.3) | 18 (78.3) | 37 (86.1) | 0.419 |
| Cardiovascular disease, n (%) | 25 (37.9) | 6 (26.1) | 19 (44.2) | 0.149 |
| Coronary artery disease, n (%) | 16 (24.2) | 1 (4.4) | 11 (25.6) | 0.033 |
| Diabetes mellitus, n (%) | 25 (37.9) | 7 (30.4) | 18 (41.9) | 0.362 |
| Peripheral arterial disease, n (%) | 12 (18.2) | 1 (4.4) | 11 (25.6) | 0.033 |
| COVID infection, n (%) | 11 (16.7) | 5 (21.7) | 6 (14.0) | 0.419 |
| Cerebrovascular disease, n (%) | 9 (13.6) | 1 (4.4) | 8 (18.6) | 0.108 |
| Depression, n (%) | 10 (15.2) | 6 (26.1) | 4 (9.3) | 0.070 |
| Cancer, n (%) | 6 (9.1) | 2 (8.7) | 4 (9.3) | 0.935 |
| CCI score, median (IQR) | 5 (2.75–7.00) | 3 (2–5) | 6 (4–8) | 0.002 |
| Patients on immunosuppressant drugs, n (%) | 2 (3.0) | 2 (8.7) | 0 (0) | 0.050 |
| Patients on psychotropic drugs, n (%) | 26 (39.4) | 9 (39.1) | 17 (39.5) | 0.974 |
| Patients on vitamin D supplementation, n (%) | 50 (76.8) | 19 (82.6) | 31 (72.1) | 0.343 |
| Laboratory evaluation | ||||
| Hemoglobin (g/dL), mean ± SD | 10.6 ± 1.2 | 10.6 (1.2) | 10.7 (1.2) | 0.633 |
| Urea (mg/dL), mean ± SD | 120.81 ± 29.9 | 130.5 (31.1) | 115.7 (27.7) | 0.062 |
| Albumin (g/dL), mean ± SD | 3.5 ± 0.4 | 3.5 (0.3) | 3.7 (0.4) | 0.256 |
| Sodium (mmol/L), mean ± SD | 138.1 ± 3.4 | 138.6 (3.6) | 137.9 (3.2) | 0.358 |
| C-reactive protein (mg/L), mean ± SD | 8.9 ± 15.4 | 4.1 (3.9) | 11.3 (18.1) | 0.378 |
| Bicarbonate (mmol/L), mean ± SD | 23.8 ± 2.8 | 22.8 (3.4) | 24.4 (2.3) | 0.050 |
| Intact parathyroid hormone (pg/mL), mean ± SD | 496.4 ± 239.6 | 419.8 (211.6) | 537.4 (240.9) | 0.062 |
| Vitamin D (ng/mL), mean ± SD | 18.6 ± 9.6 | 20.3 (10.7) | 17.3 (8.0) | 0.702 |
CCI: Charlson comorbidity index; COVID: coronavirus disease; IQR: interquartile range; MCI: mild cognitive impairment; MOCA: Montreal cognitive assessment; SD: standard deviation. Statistical significance P < 0.05.
About 42% of our population had education of 9 years or less; 38% were professionally active. Almost a quarter (23%) had the need of a helper to perform PD treatment. Mean PD therapy vintage was 24.0 ± 20.0 months. Automated PD (APD) was the most common modality (56%). During a 6-month period, we recorded 15 episodes of peritonitis and two exit-site infections.
Cognitive function
Of the 66 patients, 43 (65%) fulfilled the criteria of MCI by MOCA. Median score was 23.5 (IQR 21.0–27.0). Patients with MCI had impairment of all evaluated areas, which were as follows: visuospatial/executive, naming, attention, language, abstraction, delayed recall, and orientation.
In MMSE test, 22 (33%) patients had a score compatible with MCI. Median score was 27.0 (IQR 24.0–29.0). Similar to MOCA test, all cognitive areas evaluated by MMSE were impaired in MCI patients, which were as follows: orientation, immediate and delayed recall, attention and calculation, language, and visuospatial.
MCI assessed by assistant nephrologists was detected in 24 (36%) of patients. This detection correlated with the results obtained by MMSE, but not by MOCA test (P = 0.003 and P = 0.850, respectively).
Risk factors for MCI by MOCA test
In univariate analysis (Mann–Whitney test and Chi-square test), patients with MCI detected by MOCA test were older (P < 0.001) and less professionally active (P < 0.005), had higher body mass index (P < 0.014) and lower levels of education (P < 0.005). The Charlson comorbidity score was higher in the MCI group (P < 0.002), with a higher prevalence of vascular diseases such as coronary artery disease (CAD) (P < 0.006) and peripheral arterial disease (PAD) (P < 0.033).
Regarding PD treatment [Table 2], patients with MCI were more dependent on a helper to perform the treatment (P < 0.009). Continuous ambulatory PD (CAPD) modality was more common (P < 0.019). A lower weekly Kt/V (2.2 vs. 2.6, P < 0.012) and a lower nPCR (0.8 vs. 1.1 g/kg, P < 0.001) were observed in the MCI group. No statistical difference was found in residual renal function.
| Variable | Total n = 66 | Control n = 23 | MCI by MOCA test n = 43 | P |
|---|---|---|---|---|
| PD vintage (months), mean ± SD | 24.0 (20.0) | 20.7 (16.2) | 25.4 (21.1) | 0.457 |
| Automated PD, n (%) | 33 (56.1) | 16 (69.6) | 21 (48.8) | 0.019 |
| Helper-assisted PD, n (%) | 15 (22.7) | 1 (4.4) | 14 (32.6) | 0.009 |
| Overhydration by BIA, n (%) | 23 (34.9) | 7 (30.4) | 16 (37.2) | 0.582 |
| Peritonitis, n (%) | 15 (22.7) | 2 (8.7) | 13 (30.2) | 0.047 |
| Exit-site infection, n (%) | 2 (3.0) | 1 (4.4) | 1 (2.3) | 0.648 |
| RRF (mL/min), mean ± SD | 4.8 (3.4) | 5.6 (4.7) | 4.4 (2.4) | 0.930 |
| Kt/V, mean ± SD | 2.3 (0.4) | 2.6 (0.7) | 2.2 (0.3) | 0.012 |
| ClC (L/week), mean ± SD | 76.1 (29.6) | 88.2 (40.0) | 70.7 (20.7) | 0.239 |
| nPCR (g/kg), mean ± SD | 0.9 (0.3) | 1.1 (0.4) | 0.8 (0.2) | 0.000 |
BIA: bioimpedance analysis; ClC: creatinine clearance; MCI: mild cognitive impairment; MOCA: Montreal cognitive assessment; nPCR: normalized protein catabolic rate; PD: peritoneal dialysis; RRF: residual renal function; SD: standard deviation. Statistical significance P < 0.05, K = dialyzer blood water urea clearance, t = dialysis session length, V = distribution volume of urea.
On multivariable analysis [Table 3], level of education, Kt/V, and nPCR were found to be strongly associated with MCI (P = 0.029, P = 0.037, and P = 0.019, respectively).
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age | 1.03 | 0.95–1.11 | 0.499 |
| Education | 4622.72 | 2.34–9.12e+7 | 0.029 |
| Kt/V | 0.01 | 2.16e-4–0.71 | 0.037 |
| ClC | 1.11 | 0.99–1.24 | 0.068 |
| nPCR | 1.90e-9 | 9.43e-17–5.51e+17 | 0.019 |
CI: confidence interval, ClC: creatinine clearance, MCI: mild cognitive impairment, nPCR: normalized protein catabolic rate, OR: odds ratio. Logistic regression test. Statistical significance P < 0.05, K = dialyzer blood water urea clearance, t = dialysis session length, V = distribution volume of urea.
Risk factors for MCI by MMSE test
Patients with MCI detected by MMSE test were older (P < 0.013) and more dependent on a helper (P = 0.002). Similar to the results obtained by MOCA test screening, CCI score was higher in the MCI group (P < 0.001), with a higher prevalence of cardiovascular disease (P = 0.048), PAD (P = 0.007), and also diabetes (P = 0.048).
About 86% of patients with MCI detected by MMSE also satisfied the MCI criteria by MOCA test. Scores obtained by MMSE test [Figure 1] correlated with those of MOCA test (r = 0.72, P < 0.001).
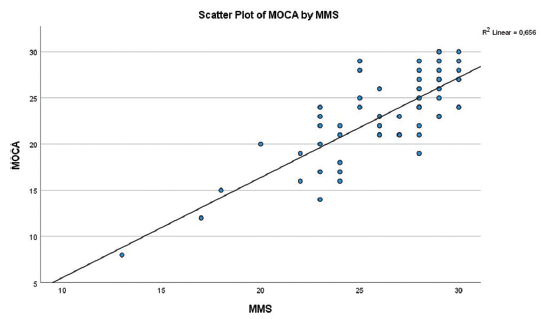
- Scatter plot of MOCA and MMSE test. MMSE: Mini Mental State Examination; MOCA: Montreal Cognitive Assessment.
No statistical difference was found regarding PD modality, dialysis adequacy, or nutritional parameters in the MCI group.
Helper versus self-care PD
Patients with the need of a helper to perform PD were older (P = 0.001), had a lower level of education (P = 0.014), and presented with a higher CCI score (P = 0.003). They had lower scores in MOCA test (P = 0.004) at the expense of impairment of visuospatial/executive and language areas [Table 4]. However, there was no statistical difference in infection episodes such as exit-site infection or peritonitis.
| Total n = 66 | Helper n = 15 | Self-care PD n = 51 | P | |
|---|---|---|---|---|
| Total score, median (IQR) | 23.5 (21–27) | 21 (17–23) | 24 (21–27) | 0.004 |
| Visuospatial/executive, median (IQR) | 3 (2–4) | 2 (1–3) | 4 (3–4) | 0.002 |
| Naming, median (IQR) | 3 (2–3) | 3 (2–3) | 3 (2–3) | 0.502 |
| Attention, median (IQR) | 5 (4–5.25) | 5 (3–5) | 5 (4–6) | 0.090 |
| Language, median (IQR) | 3 (2–3) | 2 (1–2) | 3 (2–3) | 0.003 |
| Abstraction, median (IQR) | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.190 |
| Delayed recall, median (IQR) | 2.5 (1–4) | 2 (1–4) | 3 (1–4) | 0.380 |
| Orientation, median (IQR) | 6 (5–6) | 6 (5–6) | 6 (6–6) | 0.068 |
IQR: interquartile range, PD: peritoneal dialysis. Statistical significance P < 0.05
Peritonitis events
During a 6-month period, 15 patients had at least one episode of peritonitis; one patient met the criteria for recurrent peritonitis and another one for relapsing peritonitis. Gram-positive bacteria were more frequent (50%) and 25% had culture-negative peritonitis.
MCI detected by MOCA test was associated with peritonitis (2 vs. 13 episodes in the control and MCI groups, respectively, P = 0.047); however, no statistical difference was obtained when using MMSE test. There was no statistical difference between peritonitis events and PD modality.
Regarding outcomes, three patients had to remove PD catheter, two of which were transferred to HD; the remaining patients recovered and continued with their PD treatment.
Discussion
Our PD population consisted of 66 patients, with a mean age of 60 years; most of them were male and had a high burden of comorbidities. MCI prevalence assessed by MOCA and MMSE test was 65% and 33%, respectively. In our population, patents with MCI assessed by both tests were older, with a higher Charlson index, and were dependent on a third person to perform their dialysis treatment. MCI obtained by MOCA correlated with peritonitis events.
MOCA and MMSE testing gave a different prevalence of the problem in our population, and this reflects the greater sensitivity of MOCA test, when compared to MMSE, in detecting early changes in cognitive function,1,5,6,23 and it seems that MOCA would be the preferable test for detecting MCI in PD patients. Even so, we obtained a reasonable correlation [Figure 1] between MOCA and MMSE scores, since 86% (n = 19 of 22) of patients with MCI detected by MMSE also met the MCI criteria with MOCA test.
Similar to Kwan et al.,10 we questioned the two nephrologists responsible for the patients enrolled in the study about whether or not their patients would have MCI. We found that the answer given by the nephrologists correlated with the results obtained by MMSE, but not MOCA test. This means that, if the patient’s physician is not able to detect the existence of MCI, or is only able to detect it at an advanced stage, the time window for implementing measures that can reduce progression of MCI (e.g., referring the patient for a more detailed neurologic evaluation or attempting to reduce the adverse outcomes associated with MCI) may be missed.11 This also means that routine application of global cognition tests such as MOCA test should be applied in clinical practice.
When detected by MOCA test, patients with MCI were older, had lower levels of education, and were less professionally active, characteristics that have already been identified as risk factors for MCI.5,11 We found no statistical difference regarding gender. These patients had a greater number of associated diseases, particularly vascular disease, than the control group. Vascular dysfunction appears to be an important cause of morbidity in patients with chronic kidney disease and seems to contribute to MCI through changes in macro- and microcirculation, promoting endothelial dysfunction and reduced cerebral blood flow.9
Patients who needed a helper performed worse in MMSE and MOCA testing. Compared to the control group, these patients had impairment of visuospatial/executive and language areas. Although all cognitive areas are necessary to perform daily tasks, area of executive function seems to be essential for carrying out PD treatment in a safe manner.19 Language impairment can also interfere with the patient’s ability to understand his/her treatment.24 Dysfunction in the visuospatial and executive areas, together with memory area, is associated with a greater rate of error in performing the PD technique and a greater probability of developing peritonitis.4,6,11
Assisted PD allows patients with MCI to carry out their treatment without a greater risk of adverse effects.4 This was found in our study also, in which there were no more episodes of peritonitis in this population. We also tried to understand whether in patients without a helper, there would be an association between episodes of peritonitis and MCI; despite a trend toward more infections in patients with MCI (seven episodes of peritonitis in patients with MCI vs. only two in patients without MCI), we were unable to obtain a significant statistical value (data not shown). Regardless of the results, detection of patients that might benefit from a helper can be beneficial to reduce negative outcomes, such as technique failure, peritonitis, hospitalization, and death.
In our population, we detected 15 peritonitis events and, although it seemed to exist a relationship between these episodes and MCI detected by MOCA test, we did not obtain this statistical difference with MMSE test. Gram-positive bacteria were more frequent and may be associated with contamination by skin microorganisms and breach of aseptic technique.4 Identification of MCI may help to carry out preventive measurements to reduce these events.
In PD patients, previous episodes of peritonitis or exit-site infection, or exposure to toxic elements caused by glucose-based dialysate can contribute to a chronic inflammatory state.1 An approach to treat these patients, with medication that could target inflammation, still needs to be developed.9
Regarding PD modality, 56% of our population underwent APD. However, those with MCI were mostly in CAPD. CAPD modality requires a greater number of exchanges than APD and some argue that, because of it, might have higher risk of technique failure and connection contamination.4 A recent study had shown that patients with MCI on CAPD treatment had a higher risk for developing peritonitis and a lower rate of survival.18 In our population, we did not observe a relationship between infection-related PD events and the type of PD (data not shown). Transition to APD could, however, be proposed as a measure to potentially reduce the adverse outcomes related to infection and mortality in targeted patients with MCI, but learning how to operate with a machine can be an obstacle in MCI patients.
Regarding PD treatment, we found that patients with MCI had lower Kt/V values compared to the control group. Lower levels of Kt/V translate into lower solute clearance and perpetuation of uremic environment. It should be noted that the values discussed are >1.7 (2.2 vs. 2.8), defined by Kidney Disease Outcomes Quality Initiative (KDOQI) as the “minimal delivery dose” to adequate dialysis.25 As such, it is difficult to understand the true impact of uremia on the cognitive function of these patients. There are several studies that demonstrate the relationship between uremia and the decline in cognitive function, through various pathologic mechanisms that contribute to neuroinflammation and neurotoxicity.8,9,26 The relationship between dialysis adequacy and cognitive function has been evaluated in some small studies, mostly in HD.27
Furthermore, the results are far from being consensual, and not all studies show a positive association between Kt/V and cognitive function.22,28,29
Also, we found that MCI patients presented with lower values of nPCR. Low levels of nPCR (particularly, <0.8 g/kg) seem to be associated with increased mortality in patients on PD.1,30 It was reported that low levels of nPCR, albumin, creatinine, and phosphorus can be associated with malnutrition and frailty,5,7 which can be a risk factor associated with MCI.
The strengths of our work are a reasonable number of PD patients and the use of two different tests to assess MCI. It also has some limitations: it is a cross-sectional, single-center study, and so, we cannot infer causal relationship; the majority of our patients were in APD modality, which may not be the truth in other clinical settings and may affect the external validity of the results; also, confounding variables may have interfered with the results.
Conclusion
In our PD population, MCI was detected in 65% and 33% by MOCA and MMSE test, respectively. MCI patients were older, with lower education levels and higher disease burden. Lower Kt/V and nPCR levels were associated with MCI. We also found that MCI patients had more episodes of peritonitis, although more investigation is needed to establish this association.
Acknowledgement
We thank Centro Hospitalar Universitário Lisboa Central (CHULC) for approval of this study. We thank the patients for their consent to participate in the study.
Conflicts of interest
There are no conflicts of interest.
References
- Malnutrition-inflammation is a risk factor for cerebral small vessel diseases and cognitive decline in peritoneal dialysis patients: A cross-sectional observational study. BMC Nephrol. 2017;18:1-10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cognitive impairment in non-dialysis-dependent ckd and the transition to dialysis: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2018;72:499-508.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors for mortality in patients undergoing peritoneal dialysis: A systematic review and meta-analysis. Ren Fail. 2021;43:743-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The association of cognitive impairment with peritoneal dialysis-related peritonitis. Perit Dial Int. 2019;39:229-35.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of mild cognitive impairment in automated peritoneal dialysis patients. Nephrol Dial Transplant. 2021;36:2106-11.
- [CrossRef] [PubMed] [Google Scholar]
- Self-care peritoneal dialysis patients with cognitive impairment have a higher risk of peritonitis in the second year. Perit Dial Int. 2019;39:51-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive changes in peritoneal dialysis patients: A multicenter prospective cohort study. Am J Kidney Dis. 2018;72:691-700.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive disorders in patients with chronic kidney disease: Specificities of clinical assessment. Nephrol Dial Transplant. 2021;37:ii23-32. doi: 10.1093/ndt/gfab262
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol. 2020;16:452-69.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence, types and recognition of cognitive impairment in dialysis patients in South Eastern Sydney. Intern Med J. 2021;51:2034-41.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of cognitive impairment among peritoneal dialysis patients: A systematic review and meta-analysis. Clin Exp Nephrol. 2019;23:1221-34.
- [CrossRef] [PubMed] [Google Scholar]
- The comparison of cognitive function and risk of dementia in CKD patients under peritoneal dialysis and hemodialysis: A PRISMA-compliant systematic review and meta-analysis. Med (United States). 2019;98:e14390.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors and clinical outcomes of cognitive impairment in diabetic patients undergoing peritoneal dialysis. Kidney Blood Press Res. 2021;46:531-40.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal dialysis is associated with better cognitive function than hemodialysis over a one-year course. Kidney Int. 2018;93:430-8.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of dialysis modality choice on cognitive functions in patients with end-stage renal failure: A systematic review and meta-analysis. Int Urol Nephrol. 2021;53:155-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cognitive dysfunction and health-related quality of life in patients with end-stage renal disease undergoing hemodialysis in comparison with patients undergoing peritoneal dialysis: A cross-sectional study. Med Sci Monit. 2021;28:1-13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cognitive impairment in end stage renal disease patients undergoing hemodialysis: Markers and risk factors. Int J Environ Res Public Health. 2022;19:2389.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence and prognosis of coexisting frailty and cognitive impairment in patients on continuous ambulatory peritoneal dialysis. Sci Rep. 2018;8:1-10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cognitive impairment in peritoneal dialysis patients. Am J Case Rep. 2011;57:612-20.
- [PubMed] [PubMed Central] [Google Scholar]
- Does dialysis modality affect the development of cognitive impairment? Kidney Int. 2018;93:306-8.
- [CrossRef] [PubMed] [Google Scholar]
- A survey of cognitive function in peritoneal dialysis patients. Ther Apher Dial. 2022;26:822-6.
- [CrossRef] [PubMed] [Google Scholar]
- Association between dialysis adequacy and cognition in patients with peritoneal dialysis. Psychiatry Investig. 2020;17:1143-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cognitive assessment tools for mild cognitive impairment screening. J Neurol. 2021;268:1615-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cognition in chronic kidney disease: A systematic review and meta-analysis. BMC Med. 2016;14:1-10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical practice guidelines for peritoneal dialysis adequacy guideline 1. Initiation of dialysis. Am J Kidney Dis Suppl. 2006;48:S99-102.
- [CrossRef] [PubMed] [Google Scholar]
- “Is it removed during dialysis?”—Cognitive dysfunction in advanced kidney failure—Areview article. Front Neurol. 2021;12:1-18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence and correlates of cognitive impairment in hemodialysis patients: The frequent hemodialysis network trials. Clin J Am Soc Nephrol. 2010;5:1429-38.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Associations between small and middle molecules clearance and the change of cognitive function in peritoneal dialysis. J Nephrol. 2020;33:839-48.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cognitive function and dialysis adequacy: No clear relationship. Am J Nephrol. 2011;33:33-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Relationship of normalized protein catabolic rate with nutrition status and long-term survival in peritoneal dialysis patients. Adv Perit Dial. 2015;31:45-8.
- [PubMed] [Google Scholar]







