Translate this page into:
Detections of Chemicals and Migratory Plastics in Peritoneal Dialysis Fluids
Corresponding author: Sanjay Kumar Panda, Department of Nephrology, Base Hospital Delhi Cantt, New Delhi, India. E-mail: subhrasanjay@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Panda SK, Maloth RK, Upadyayalu V, Mishra A, Noronha S. Detections of Chemicals and Migratory Plastics in Peritoneal Dialysis Fluids. Indian J Nephrol. 2025;35:34-9. doi: 10.25259/ijn_515_23
Abstract
Background:
Peritoneal dialysis (PD) is an important modality of renal replacement therapy (RRT). Peritonitis and ultrafiltration failure are complications that have a long-term impact on PD patients. Besides touch contamination, procedural errors and clinical reasons of peritonitis, contaminants, and constituents of peritoneal dialysis fluids (PDFs) have been implicated in causing peritonitis and ultrafiltration failure. This study was aimed to test the PDFs in India for the presence of migratory plastics.
Materials and Methods:
PDFs from the two manufacturers in India were tested using liquid chromatography mass spectrometry (LCMS) and gas chromatography mass spectrometry (GCMS) with headspace analysis (volatile compounds) and pyrolysis of plastics (polymer compounds). The storage conditions and handling were uniform.
Results:
The results revealed impurities of acetate compounds and aldehyde derivatives of glucose degradation products (GDPs) with contaminants and leachable plastics. There were high levels of GDP derivative in the form of 5-hydroxymethylfurfural compounds (5-HMF). The analysis revealed the presence of plastic softeners in very high concentrations.
Conclusion:
The study unmasks the presence of chemicals and GDPs that can be implicated in pathogenesis of sterile peritonitis and ultrafiltration failure. The study demonstrated the presence of leachable plastics. In conclusion, LCMS and GCMS studies can be used to test PDFs for unwanted chemicals prior to human use.
Keywords
Peritoneal dialysis fluids
Chemical peritonitis
Ultrafiltration failure
Glucose degradation products
Renal replacement therapies
Introduction
Chronic kidney disease (CKD) is a global pandemic.1 Despite better preventive strategies and advances in treatment, the number of patients reaching end-stage kidney disease (ESKD) are increasing and these patients require renal replacement therapies (RRT).2 The burden of ESKD patients across the world is increasing and in India it was observed that approximately the third of the CKD population are progressing to ESKD annually.3 In view of a perpetual lack of organs for transplant, other forms of RRTs have an important place in ESKD therapy.4 However, hemodialysis has infrastructure requirements and is limited by finite capacity, hence peritoneal dialysis (PD) is a viable alternative.5 The benefits of an early start PD program, especially when residual kidney function (RKF) is present are well documented.5 The success of PD first policy as instituted by the South East Asian countries is proof of the concept and needs to be emulated by other developing nations.6 The twin threats to a successful PD program are peritonitis and ultrafiltration failure.7 Several factors have been ascribed to these events, such as technique failure due to poor training or non-compliance to the recommended precautions for the prevention of contamination as well as long-term effects of osmotic agents (glucose) on the peritoneal membrane.8,9 The clinical outcome of various brands of PDFs is not documented in India due to various factors. First, due to the lack of a nationwide registry, outcomes with specific brands of PDF are not available. Second, although Indian Pharmacopoeia 2018 has stated the requirement for high performance liquid chromatography (HPLC) data for certification of PDFs but, manufacturers are getting clearance from drug regulatory authorities based on the chemical composition of dextrose, sodium, and lactate.10 Private industrial laboratories perform these tests in the absence of a central facility. Third, there are no existing guidelines in the country regarding the PDF quality. Among the varied etiologies for sterile peritonitis, chemical peritonitis can be due to PDFs composition and there are investigative reports in literature where PDFs have been implicated as the cause of peritonitis due to defects in manufacturing processes.11-15 These studies were based on HPLC to detect glucose degradation products (GDPs) or endotoxin assays to detect bacterial contamination.11-15 The deleterious effect of GDPs on the peritoneal membrane viability is well documented.16 The occurrence of culture negative peritonitis in the country is well above the 20% benchmark set by International Society of Peritoneal Dialysis (ISPD) guidelines and hence, it is imperative to have a relook at the PDFs composition based on liquid chromatography mass spectrometry (LCMS) and gas chromatography mass spectrometry (GCMS)-based platforms.17,18 The present study is a cross-sectional analysis of PDFs available in India using both LCMS- and GCMS-based technologies to determine the chemical content and presence of plastic precursors in PDFs.
Materials and Methods
Random samples of different batches of PDFs from the two different manufacturers were collected from the hospital stores. The bags were stored in the hospital warehouse and subsequently at 4°C till tested. The fluids from both manufactures were labeled as samples A1, A2 and samples B1, B2, respectively. One batch of PD fluids (A1 and B1) consisted of 1.5% dextrose, and another batch of PD fluids (A2 and B2) consisted of 2.5% dextrose. Multiple bags from the batches were tested. The HPLC grade solvents dichloromethane, ethyl acetate, optima LC-MS grade methanol and acetonitrile were purchased from Thermo Fisher Scientific (Mumbai, India). As the fluid were used as standard of care therapy and no patients were involved, there was no need for ethical clearance or patient consent.
Sample Preparation: GCMS and LCMS Study
The samples were subjected to different sample preparation procedures for the analysis. The presence of volatile and semi-volatile impurities and the identification of various degradation products of glucose and other ingredients present in the PDFs were carried out by solvent extraction process. The detailed process for sample preparation is provided in Supplementary File 1.
GC-MS analysis
GC-MS analysis of volatiles and semi-volatiles. The GC-MS analysis of volatiles and semi-volatiles components present in the dichloromethane extracts of the PDFs and methanolic extracts of the plastic bags and tubing was conducted on Agilent 5977 A mass selective detector coupled with Agilent 7890 GC and G4513A auto sampler. The analytes were identified by NIST14 library database.
Headspace GC-MS analysis (Hs-GC-MS). The HS-GC-MS analysis was conducted on Agilent 5973N mass selective detector coupled to Agilent 6890 GC system and G1888 Headspace sampler. The analytes were identified by NIST14 library database.
Pyrolysis GC-MS analysis (Py-GC-MS). The pyrolysis GC-MS analysis was conducted on Agilent 7000D triple quadruple mass spectrometer connected to Agilent 8890B GC system and Frontier Lab EGA/PY-3030D multishot pyrolizer system.
The detailed process for GCMS analysis is provided in Supplementary File 2.
LC-MS analysis
The LC-MS analysis was carried out on a Thermo QExactive Orbitrap mass spectrometer connected to Quaternary gradient Vanquish Ultra performance liquid chromatography system and auto sampler.
The detailed process for LCMS analysis is provided in Supplementary File 3.
Results
GC-MS and HS-GCMS Analysis
The results showed the presence of cyclohexanone, 2-ethyl-1-hexanol, benzyl alcohol, 1,2,3,5-tetramethylbenzene, 2,4-di-tert-butylphenol and di-butylphthalide, in the analyzed samples. The samples A1 and A2 exclusively revealed the presence of furfural, acetophenone, 2-5-furandicarboxaldehyde, benzylalcohol, isobenzofuranone, and 2-ethylhexyl phthalic acid, which were not detected in B1 and B2 samples. The comparative chromatograms of A1 and B1 samples are presented in Figure 1. The chemicals identified in all four analyzed samples are presented in Table 1. The HS-GCMS analysis did not reveal the presence of any exclusive chemicals other than cyclohexanone which was commonly observed in all the collected samples. This may be due to the degradation of some of the volatiles due to high temperature in the oven of the headspace sampler.
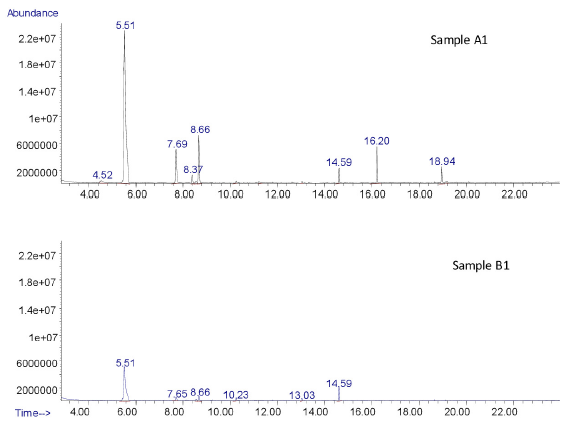
- Total ion chromatograms indicating the difference between the volatile chemicals present in samples A1 and B1.
| Name of Chemical | Ret. Time (min) | Sample A1 | Sample A2 | Sample B1 | Sample B2 |
|---|---|---|---|---|---|
| Furfural | 4.52 | Yes | Yes | No | No |
| Cyclohexanone | 5.51 | Yes | Yes | Yes | Yes |
| 2-Ethyl-1-hexanol | 7.69 | Yes | Yes | Yes | Yes |
| Acetophenone | 8.37 | Yes | Yes | No | No |
| 2,5- Furandicarboxaldehyde | 8.53 | Yes | Yes | No | No |
| Benzyl alcohol | 8.66 | Yes | Yes | Yes | Yes |
| 1,2,3,5-Tetramethylbenzene | 9.22 | Yes | Yes | Yes | Yes |
| 1(3H)-isobenzofuranone | 12.61 | Yes | Yes | No | No |
| 2,4-di-tert-butyl phenol | 14.59 | Yes | Yes | Yes | Yes |
| Dibutyl phthalate | 18.63 | Yes | Yes | Yes | Yes |
| 2-ethylhexyl phthalic acid | 18.94 | Yes | Yes | No | No |
Ret.: Retention
Pyrolysis-GC-MS Analysis of Plastic Bags
The volatiles obtained from the pyrolysis GC-MS analysis showed the presence of butylated hydroxytoluene, bis(2-ethylhexyl) adipate, and bis(2-ethylhexyl) phthalate. These three were commonly observed in the bags of both manufacturers. However, the peak height and area of bis(2-ethylhexyl) phthalate is more in samples B1 and B2, indicating more smoother texture as compared to that of A1 and A2. The comparative Py-GC-MS chromatograms are represented in Figure 2. The difference between the bags of the two manufacturers is clearly visible in Figure 2. The Py-GC-MS analysis of the tubing also showed that the bags and tubing were made with a similar kind of material and the chromatograms are reproducible indicating the same compounds.
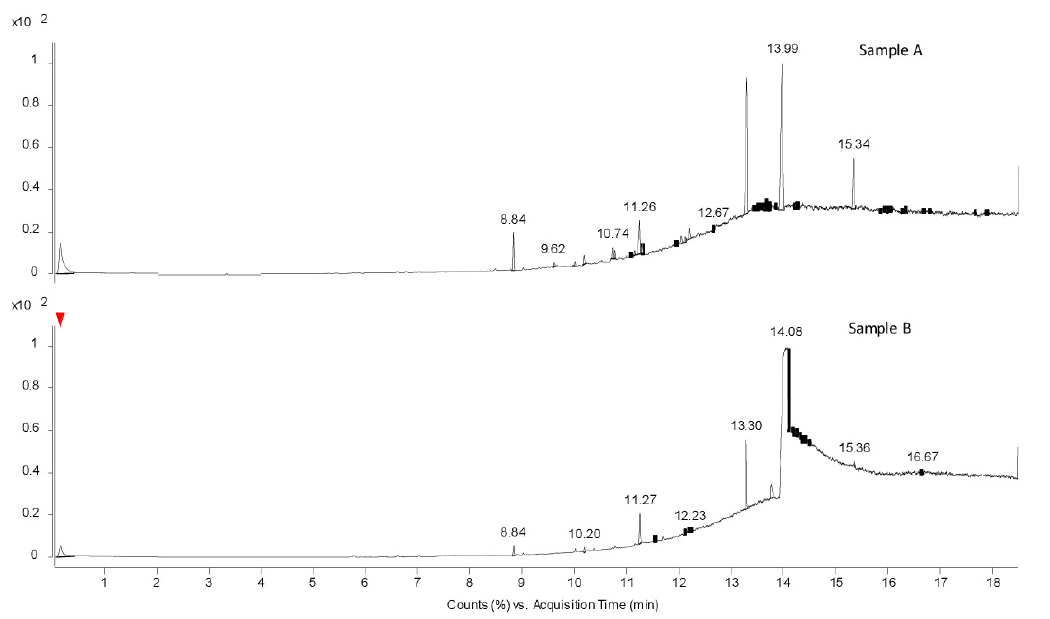
- Thermal desorption chromatograms obtained from Py-GC-MS in samples A and B.
Migrating chemicals from the bags and tubing by GC-MS
The results showed the presence of various analytes as listed in Table 2. From the table, it can be clearly observed that the plastic bags/tubes used for packing sample A contains a greater number of leachable as compared to that of sample B.
| Name of Chemical | Ret. Time (min) | Sample A | Sample B |
|---|---|---|---|
| Cyclohexanone | 5.4 | Yes | Yes |
| 2-Ethyl-1-hexanol | 7.65 | Yes | Yes |
| Acetophenone | 8.32 | Yes | No |
| Phenyl-tert-butanol | 8.61 | Yes | No |
| Nonanal | 8.88 | Yes | No |
| 2-Ethylhexylpentanoate | 13.36 | Yes | No |
| 2,4-Di-tert-butylphenol | 14.57 | Yes | No |
| Butylated hydroxytoluene | 14.65 | Yes | Yes |
| 2-Ethylhexylbenzoate | 16.93 | Yes | Yes |
| o-Tolueic acid, 2-ethylhexyl ester | 17.73 | Yes | No |
| Isohexyl methyladipate | 18.11 | Yes | No |
| Butylphthalate | 18.9 | Yes | Yes |
| Hexadecanoic acid | 19.45 | Yes | Yes |
| 2-Ethylhexyl isopropylphthalate | 20.96 | Yes | No |
| Pentadecyl-o-tolueate | 22.23 | Yes | No |
| Bis(2-ethylhexyl)adipate | 23.62 | Yes | No |
| Octylhexadecanoate | 24.27 | Yes | No |
| Bis(2-ethylhexyl)phthalate | 24.87 | Yes | Yes |
| Isopropyl octadecenoate, 9-epoxy | 27.86 | Yes | No |
| 9,12-Diepoxyethylstearate | 29.51 | Yes | No |
Ret.: Retention
LC-MS Analysis
Sample A
In sample A, an extra retention time (RT) around 7.61 minutes was observed in both 1.5% and 2.5% solutions. The compound had the molecular formula C7H12O3. Probably, the compound was tetrahydrofurfuryl acetate. The formation of acetate derivatives was not clear since PDFs do not contain any acetate salts. The LCMS for both 1.5% and 2.5% were similar as represented in Figure 3.
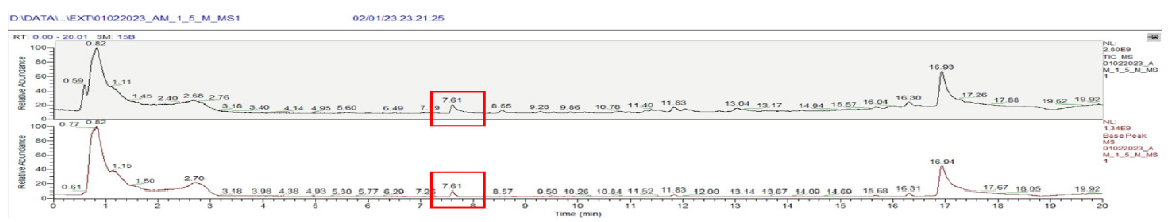
- LCMS data 1.5% sample A with compound highlighted at retention time of 7.61 minutes. The red box shows presence of abnormal compounds at retention time of 7.61 minutes. LCMS: Liquid chromatography mass spectrometry.
The relative abundance and approximate compound concentration in sample A is 0.12 parts per million (ppm) for 1.5% and 0.11 ppm for 2.5% PDFs.
In sample A, at RT around 7.595 minutes, a compound having molecular formula C10H16O5 was observed in both 1.5% and 2.5% solutions. The probable compound is dimethyl-2hydroxy-1-3cyclohexane. The compound is related to leachable plastics from the plastic packaging of sample A. The relative abundance and the approximate concentration of the compound in sample A is 12 ppm(1.5%) and 10 ppm (2.5%) PDFs, respectively.
In sample A, at RT of 2.039 minutes, a compound with molecular formula C6H6O3 was present. The probable compound is 5-hydroxyl methyl furfural (5-HMF), a degradation product of sugar (corn starch). The relative abundance and approximate concentration of the compound in sample A is 19 ppm (1.5% and 2.5%).
Sample B
Sample B only revealed the presence of a single compound. At RT of 2.039 mins in sample B (1.5% and 2.5%), a compound with molecular formula C6H6O3 was present. The probable compound is 5-HMF, a degradation product of sugar (corn starch). The relative abundance and approximate concentration of compound in Sample B (1.5% and 2.5%) is 20 ppm.
Discussion
The study highlights the importance of newer technological platforms such as LCMS and GCMS in detection of constitution anamolies in PDFs which were not been detected by routine methods employed for certification of PDFs. The LCMS study revealed the presence of acetate compound in sample A fluids that would cause peritoneal inflammation and infusion pain.19 Pedersen et al. have demonstrated that acetate is the cause of peritoneal inflammation and encapsulating sclerosing peritonitis.19
Both samples revealed the presence of 5-HMF derivatives on LCMS analysis. The levels of 5-HMFs derivatives in sample A and sample B were 19 ppm and 20 ppm, respectively. HMF is a GDP generated due to heat sterilization, storage conditions, and quality of packaging providing heat insulation.20-22 GDPs have adverse effects on peritoneal mesothelial cells, fibroblasts, neutrophils, and macrophages, including induction of apoptosis, disturbance in cytokine production along with inhibition of migration, bacterial killing, phagocytosis, and respiratory bursts in phagocytic cells.23,24 GDPs, such as 5- HMF, have lower toxicity but given the repetitive nature of the PD and ability of 5-HMF to convert to other GDPs, British Pharmacopoeia (BP) has recommended a concentration of less than 5 ppm with self-life monitoring, which is not done in India.25
GCMS analysis of sample A revealed the presence of furfuraldehyde (FFA) and other derivatives of furfural. Sample B did not have any FFA compound. GDPs, which are aldehyde derivatives, demonstrate higher toxicity to peritoneal membrane at far lower concentration and exposure time.26,27 These PDFs have been associated with fibrosis and vasculopathy of peritoneal membrane leading to the recommendation of low GDP PDFs in Japan.27-29
LCMS analysis of sample A revealed the presence of a plastic precursor compound (cyclohexane derivative). The compound has been identified as a plastic derivative leaching from the plastic packaging.30 The concentration was between 10 and 12 ppm. The threshold for leachable plastics in PDFs has not been defined but should be as low as possible.
GCMS analysis revealed significant higher levels of multitude of plastic precursors (chemical names described in results) in sample A. Sample B only revealed the presence of single plastic precursor (cyclohexanone) and in far less concentration as compared to sample A. The detection of plastic precursors in PDFs is a matter of concern and toxicology references suggest they cause skin and ocular irritation.30
GCMS also showed the presence of other chemical impurities, such as phenolic compound (details given in results), in sample A. These impurities being industrial contaminants should not exist in the PDFs and possibly be attributed to contamination in the production process.
GCMS Headspace analysis of sample A revealed the presence of many volatile chemicals whereas these were not present in sample B. The Pyrolysis GCMS analysis also highlighted the presence of migratory chemicals from the plastic packaging to the fluid in sample A. The presence of these can be due to excessive use of plastic softener chemicals. These softeners are named as endocrine disrupting chemicals (EDC) by the World Health Organization.31 The European Union has set a limit for the same at 10 mg/kg concentration for migratory plastics in medical fluids.32
The guidelines for testing quality-related aspects of PDFs in European Union and the United States, which are also adopted by South East Asian countries, include testing for osmolarity, pH, dextrose, calcium, potassium, aluminum, and HMF derivatives using a combination of physical analysis, chemical tests, HPLC and atomic absorption spectroscopy-based methods.32,33 The study findings suggest that global guidelines are not been followed in India. HPLC is not mandatory and being not available in Industrial laboratories in India, was not performed. LCMS and GCMS are technological improvements and more accessible in the present era in many CSIR labs.10,18,25
Our study has certain limitations. First, PDFs from only two manufacturers were tested. The third manufacturer whose fluid was not procured under government procurement programme due to cost issues could have a different chemical composition. Second only the available PDFs batches were tested. Third, the LCMS analysis was quantitative, while GCMS was qualitative and were performed in different institutes. Finally, the data of the PDFs analysis from manufacturers and regulatory authorities was not available to the study group. This study highlights the need for analysis of PDFs with sensitive chemical detection platforms, such as LCMS and GCMS prior to human use. These platforms are available in the country.
PDFs should be manufactured and packaged to the highest quality standards for the PD therapy to be successful in developing countries. Sample A had numerous anomalies in its constitution when tested by LCMS- and GCMS-based methods. Both fluids had high levels of 5-HMF. These methods can complement other methods for constitutional analysis of PDFs by detecting various impurities and micro plastics. The presence of micro plastics in PDFs and its local and systemic effects needs further research. PDFs should be manufactured as per the geographical requirement of the country in use (low GDP thermostable fluid for tropical and sub-tropical regions).
Data availability statement
The PDFs and packaging material were analyzed at Indian Institute of Technology, Mumbai (LCMS study) and Indian Institute of Chemical Technology (CSIR lab), Hyderabad (GCMS study). The data is available in electronic and hard copy format for review.
Acknowledgements
We are thankful to Dr. Sandeep Mahajan and Dr. Soumita Bagchi from the Department of Nephrology at the All India Institute of Medical Sciences, New Delhi, for their valuable contributions. Dr. Mahajan’s work on evaluating the hypothesis regarding GCMS/LCMS technology for detecting PDF anomalies, reviewing the data to make the topic clinically relevant. Dr. Bagchi’s meticulous proofreading of the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The lancet. 2020;395:709-33.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of chronic kidney disease in Asia: A systematic review and analysis. BMJ global health. 2022;7:e007525.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease in India: Challenges and solutions. Nephron Clin Pract. 2009;111:c197-c203.
- [CrossRef] [PubMed] [Google Scholar]
- PD First: peritoneal dialysis as the default transition to dialysis therapy. In Seminars in dialysis. 2013;26:706-13.
- [CrossRef] [PubMed] [Google Scholar]
- A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit Dial Int. 2015;35:406-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Peritoneal dialysis first policy in Hong Kong for 35 years: Global impact. Nephrol. 2022;27:787-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The current state of peritoneal dialysis. J Am Soc Nephrol. 2016;27:3238-52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Peritoneal dialysis: status report in south and south east Asia. Nephrol. 2021;26:898-906.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical causes of inflammation in peritoneal dialysis patients. Int J Nephro. 2014;2014:909373.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Indian Pharmacopoeia 2018;3:2887
- Epidemic of chemical peritonitis in patients on continuous ambulatory peritoneal dialysis: A report from Western India. Perit Dial Int. 2016;36:347-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chemical peritonitis associated with high dialysate acetaldehyde concentrations. Nephrol Dial Transplant. 2000;15:2037-40.
- [CrossRef] [PubMed] [Google Scholar]
- Sterile peritonitis in the peritoneal dialysis patient. Perit Dial Int. 2005;25:146-51.
- [CrossRef] [PubMed] [Google Scholar]
- Description of an outbreak of acute sterile peritonitis in Iran. Perit Dial Int. 2010;30:19-22.
- [CrossRef] [PubMed] [Google Scholar]
- Cytotoxic glucose degradation products in fluids for peritoneal dialysis. Iran J Pharm Res. 2011;10:113.
- [PubMed] [PubMed Central] [Google Scholar]
- Un-favourable effects of peritoneal dialysis solutions on the peritoneal membrane: the role of oxidative stress. Biomolecules. 2020;10:768.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- ISPD Peritonitis guideline recommendation: 2022. Update on prevention and treatment. Perit Dial Int. 2022;42:110-153.
- [CrossRef] [PubMed] [Google Scholar]
- Toxicology: liquid chromatography mass spectrometry. In: Mass spectrometry for clinical laboratory. Academic Press; 2017. p. :109-30.
- [Google Scholar]
- Acetate versus lactate in peritoneal dialysis solutions. Nephron. 1985;39:55-8.
- [CrossRef] [PubMed] [Google Scholar]
- Buffer transport in peritoneal dialysis. Kidney Int Suppl. 2003;64:S37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Complex technological and biological research of solutions for peritoneal dialysis. Int J Appl Pharm. 2018;10:59-67.
- [CrossRef] [Google Scholar]
- Degradation and de novo formation of nine major glucose degradation products during storage of peritoneal dialysis fluids. Scientific Reports. 2022;12:4268.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Peritoneal dialysis with solutions low in glucose degradation products is associated with improved biocompatibility profile towards peritoneal mesothelial cells. Nephrol Dial Transplant. 2004;19:917-24.
- [CrossRef] [PubMed] [Google Scholar]
- Heat sterilized PD- fluids impair growth and inflammatory responses of cultured cell lines and human leukocytes. Clin Nephrol. 1993;39:343-8.
- [PubMed] [Google Scholar]
- British Pharmacopoeia 2009;Pages 589,2279-80,2535-6
- Long-term exposure to peritoneal dialysis solutions: Effect on peritoneal membrane. Kidney Int. 2004;66:1275-65.
- [CrossRef] [PubMed] [Google Scholar]
- Biocompatibility of a new PD solution for Japan, Reguneal™, measured as in vitro proliferation of fibroblasts. Clin Exp Nephrol. 2018;22:1427-36.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal dialysis guidelines 2019 part 1 (position paper of the Japanese Society for Dialysis Therapy) Renal Replacement Therapy. 2021;7:40.
- [CrossRef] [Google Scholar]
- Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev. 2018;10:CD007554.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- PubChem compound summary for CID 8078, Cyclohexane. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Cyclohexane [Last accessed 2024 June 1]
- Collaboration between the world health organization and national institute of environmental health science. WHO library Cataloguing: Publication data 2012.
- European Pharmacopeia 35.1. Solutions for Peritoneal Dialysis. Serial No 62. Ref PA/PH/ Exp Dia/ T. Text No 0862. Available Online.
- USP- NF 2023. Peritoneal Dialysis Solutions. Available Online.







