Translate this page into:
Dietary Management in Slowing Down the Progression of CKDu
Address for correspondence: Dr. Georgi Abraham, Department of Nephrology, Madras Medical Mission Hospital, 4-A, Dr J.J Nagar, Mogappair, Chennai, Tamil Nadu, India. E-mail: abraham_georgi@yahoo.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Chronic kidney disease of unknown etiology (CKDu) is an emerging entity in the South Asian region. This predominately affects the farming community belonging to the lower socioeconomic status. CKDu being a progressive condition often leads to end-stage renal failurerequiring renal replacement therapy (RRT). Due to the high cost and limited availability of RRT in many areas of geographical locations in India and worldwide, there is an unmet need to slow down the progression of CKDu. The intestinal microbiota is different in patients with CKD, with low levels of beneficial bacteria such as Lactobacillus and Bifidobacteria. Prebiotics and probiotics modify the intestinal microbiota and thereby slow down the progression. Soda bicarbonate therapy is cheap and cost-effective in slowing down the progression of CKDu in a subset of patients. There is also evidence of the beneficial effect of N-acetyl cysteine in early stages of CKD and it should benefit CKDu also. Dietary interventions to prevent dehydration, by providing uncontaminated drinking water, sufficient protein containing diet with adequate calories, and tailored salt intake to prevent hypotension, are necessary compared to other causes of CKD. The objective is to prevent malnutrition, and uremic symptoms. Early diagnosis and prompt intervention may delay the progression of CKDu in the early stages.
Keywords
CKDu
malnutrition
nutritional therapy
Introduction
The first report by the CKD registry documentsprevalence rate of 16% for chronic kidney disease of unknown etiology (CKDu) in India with 21.3% in the southern part of India.[1] Uddanam in Srikakulam district consisting of the mandals of Kaviti, Sompeta, Kanchili, Ithapuram, Palas, and Vajrapukotturu and Prakasham district of Andhra Pradesh, Goa, and Odisha [Figure 1] have higher prevalence of CKDu especially among the younger farming population.[2] Several causative associations such as farming occupation, exposure to residual pesticides, high content of cadmium and silica in the drinking water, and genetic predisposition have been thought to be involved in the pathogenesis of CKDu.[3]

- Clockwise top left : Andra Pradesh, Goa, Odisha
Intestinal Flora among Healthy Individuals and in Patients With CKD
There is a paucity of data on intestinal microflora in South Asian population in health and disease states. The human gut harbors more than 100 trillion bacterial cells in healthy individuals, which is predominately rich in bacteria such as Lactobacillus and Bifidobacteria. Whereas in uremicpatients, higher levels of pathogens such as Clostridia, Enterobacteria sp., and Pseudomonas sp. are found, with low level of Lactobacillus and Bifidobacteria.[4] Patients with end-stage renal disease (ESRD) are also at a higher risk of Clostridium difficile–associated diarrhea.
Dysbiosis refers to an imbalance in the composition of the gut microbiome. Increased secretion and hydrolysis of urea leads to excessive formation of ammonia, thereby affecting the growth of gut commensal bacteria. Gut bacteria produces endotoxins which lead to an inflammatory response in the host organism. Moreover, protein fermentation by the gut microbes produces toxic metabolites, such as p-cresoland indoxyl sulfate.[5] P-cresol is largely conjugated to p-cresylsulfate (PCS) in the intestinal wall and subsequently to p-cresyl glucuronide in the liver.[5] In CKDu like other CKD, the barrier function of the gut is disrupted causing translocation of the endotoxins and bacterial metabolites into the systemic circulationwhich contributes to the uremic toxicity, inflammation, associated cardiovascular disease, and progression of CKD.[5] The concentration of indoxyl sulfate and PCS in the serum is negatively correlated with kidney function.[6] The colonic transit time is approximately 14 h among South Asians. However, in patients with any CKD, the transit time is further prolonged, contributing to increased absorption of uremic substances. Furthermore, vitamin K deficiency or insufficiency is common among patients with any CKD due to decreased synthesis by[78] Bacteroides fragilis, Bifidobacteria, Clostridium sp, and Streptococcus faecalis.
Hence, bacterial metabolism-based interventions may be of therapeutic importance in any CKD including CKDu [Figure 2].
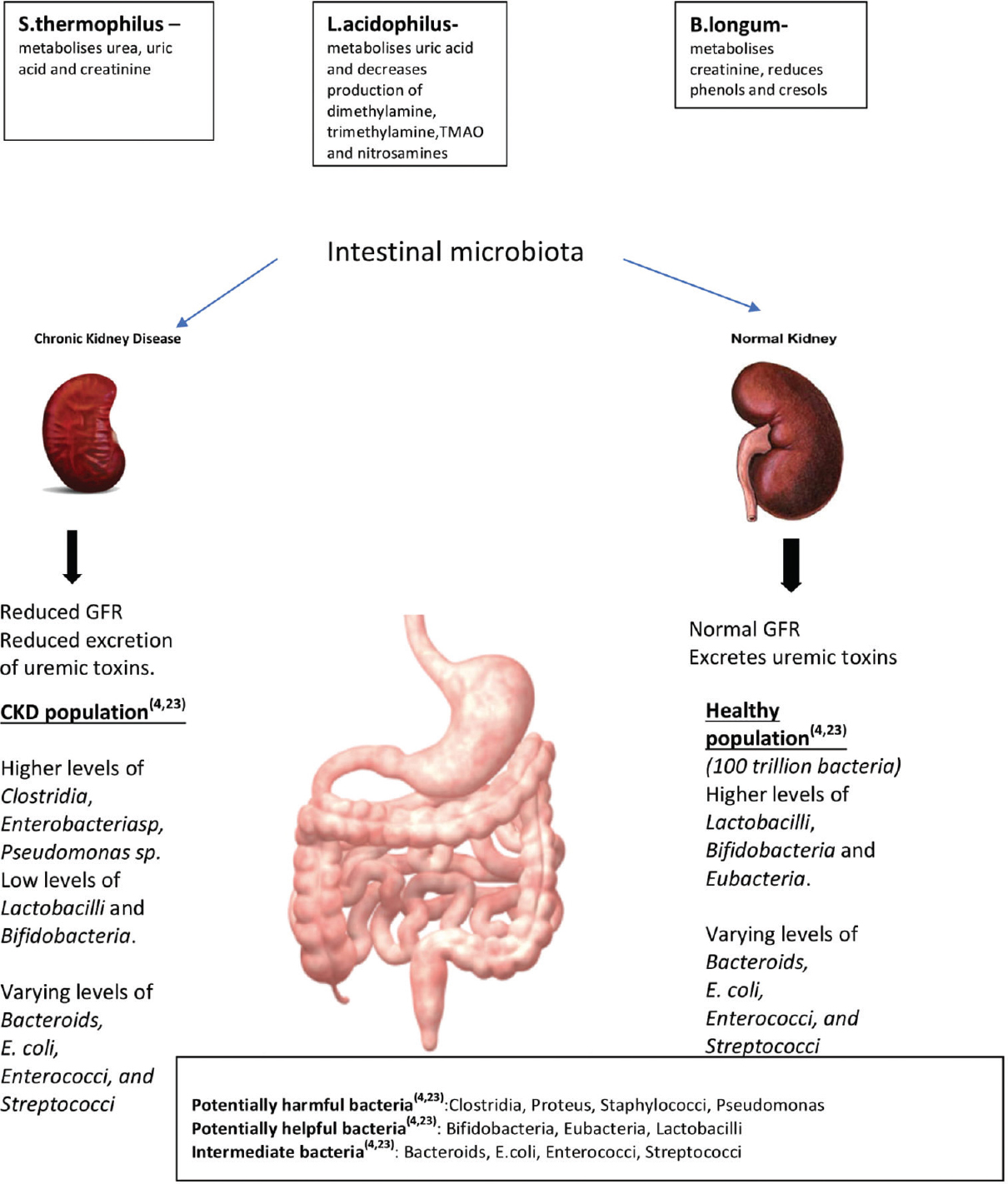
- Gut microbiota in healthy individuals and in CKD patients
Sources of prebiotics include breast milk, soybeans, raw oats, unrefined wheat, unrefined barley, non digestible carbohydrates, and in particular nondigestible oligosaccharides.[9]
The most commonly used probiotic strains are Bifidobacterium, Lactobacillus rhamnosus, Lactobacillus reuteri and certain strains of Lactobacillus casei, Saccharomycesboulardii, and Bacillus coagulans. Foods containing probiotics include fermented dairy products such as yogurt, kefir, and cheese.
Therapeutic Modulation of Gut Microbiome
Prebiotics promote growth of Bifidobacteria and Lactobacillus species, whereas they reduce certain bacteria such as Bacteroidssp, Clostridia sp, and Enterobacteria.[5] Oligofructose-enriched inulin promotes growth of Bifidobacteria sp, mediates weight loss, reduces inflammation, and improves metabolic function [Figure 2].[5] Meijers et al. reported a decrease in serum concentration of p-cresol after oral intake of oligiofructose-enriched inulin in hemodialysis patients.[10] High dietary fiber consumption lowers the risk of inflammation and reduces mortality in patients with any CKD.[11] Streptococcus thermophilus (KB19), Lactobacillus acidophilus (KB27), and Bifidobacterium longum (KB31) produce bacteriocins, namely, lactacin and bisin which inhibit the growth of pathogens,[121314] thereby reducing generation of uremic toxins. Streptococcus thermophiles metabolizes urea, uric acid, and creatinine. Bifidobacterium longumreduces the level of protein-bound uremic toxins such as phenols and cresols.
Role of Oral Bicarbonate Supplementation
Metabolic acidosis is a common complication in advanced stages of any CKD. This is often associated with increased protein catabolism due to the upregulation of the ubiquitin-proteasome system, excessive oxidation of branched chain amino acids, and reduced synthesis of visceral proteins including albumin.[15] In a randomized, prospective study in Londonby de Brito-Ashurst et al., 134 patients with creatinine clearance of 30–15 mL/min/1.73 m2 was given either oral sodium bicarbonate tablets 600 mg thrice daily dose adjusted to achieve and maintain HCO3− level ≥23 mmol/L or routine standard care. Serum bicarbonate was measured every 2 months. The rate of decline in creatinine clearance in stage 4 CKD was found to be lower among individual on bicarbonate therapy (1.88 mL/min/1.73 m2) when compared with the control group (5.93 mL/min/1.73 m2).[15] Oral bicarbonate supplementation is an affordable and accessible means of maintaining serum bicarbonate >22 mmol/L.
N-Acetyl Cysteine
N-acetyl cysteine (NAC) use reduced the risk of progression of CKD of any etiology to ESRD significantly (18%).[16] However, the mechanism by which NAC reduces the progression of CKD to ESRD is unclear. It may be attributed to the antioxidant and vasodilatory nature of NAC.[16] Prolonged duration of administration and higher dosage of NAC increase its renal protective effect.[1617] Due to its low cost, wide availability, and ease of administration, it is widely accepted.
Conservative Management Of CKDu
Consumption of uncontaminated water, avoidance of nephrotoxic drugs, chewing practices with concoction, and limited exposure to pesticides and agrochemicals[2] are some of the measures indelaying the progression of CKDu. Supportive care should include anemia correction, skilled dietician advice, and regular follow-up. Prebiotics and probiotics may be effective in partially reducing the colonic transit time. Hence, slowing the progression of CKDu. Soda bicarbonate therapy and NAC will provide value to the management of CKDu in low-resource setting.[15]
Dietary advice
As CKDu is a nonprotienuric disease, dietary advice of protein intake with 0.6 g/kg/day or a very low protein diet of 0.3 g/kg/day with ketoanlogues, 30–35 kCal/kg/day supplemented with micro nutrients may slow down the progression by reduction in serum phosphate and FGF-23 levels.[18] As in CKD low protein diet would reduce urea production, advanced glycation end products, and waste products of protein metabolism such as indoxyl sulfate, PCS, and trimethylamine N-oxide.[19]
Discussion
A review of literature showed several studies analyzing the association of prebiotics and probiotics with CKD of other known etiology but none with CKDu. Use of prebiotics and probiotics has been proven to slow down the progression of CKD, and the same may be applied in patients with CKDu.
Ranganathan conducted a 6-month prospective clinical trial assessing the biochemical and clinical effects of an oral probiotics [90 billion colony-forming units (CFUs)] in 16 stage 3 and stage 4 patients with CKD, out of which 13 completed the study. The major outcome included a reduction in blood urea nitrogen (BUN), serum creatinine, and uric acid.[20]
Similarly, Paola Vanessa et al. conducted a study on the effect of probiotics on BUN levels in 30 patients with stage 3 and stage 4 CKD. The study was aimed to determine the effectiveness of two dosing of Lactobacillus caseiShirota, 8 × 109 CFUs and 16 × 109 CFUs in achieving a decrease in the serum urea concentration. Patients with CKD show an increase in aerobic bacteria which produce uremic toxins in the bowel, with decreased anaerobic bacteria such as Lactobacillus and Bifidobacteria. Patients were followed up for 8 weeks on the probiotic supplementation. A decrease of more than 10% was found in the concentration ofblood urea and serum creatinine, noted in the population treated with higher dose of probiotics.[21]
Rathi et al. conducted a study administering enteric coatedgelatin capsules containing lyophilized Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterium longum in a dose of 15 million CFUs along with lactitol monohydrate as prebiotic to 30 predialysis CKD patients. The results showed significant improvement in various parameters of CKD.[22]
Jia et al. conducted a meta-analysis that showed probiotics supplementations may reduce the levels of PCS and elevate the levels of IL-6 which are found to be benificial.[2324]
According to the WHO and the FAO (2002), “Probiotics may theoretically be responsible for 4 types of side effects such as systemic infections, deleterious metabolic activities, excessive immune stimulation in susceptible individuals and gene transfer.”
Most patients with CKDu belong to lower socioeconomic status and they have chronic tubulointerstitial disease. Tubulointerstitial disease which is progressive; dehydration and salt loss is highly probable, and protein energy malnutrition with micronutrient deficiencies are not addressed in this CKDu lower socioeconomic strata population. Dietary advice targeted to this population is an important critical component of the treatment. Patients with CKDu are at high risk of dehydration and dehydration-related acute on chronic kidney injury due to their tubulointerstitial diseases. Special diet catering to the communities where CKDu prevalence is high, providing appropriate calories and proteins as per stage of CKD, should be emphasized.[2526]
Conclusion
Prebiotics and probiotics alter the microbiome of the gastrointestinal system, thereby reducing the production of uremic toxins, aid in slowing down the progression of CKD. Soda bicarbonate therapy ameliorates any CKD progression. Nutritional monitoring and targeted nutritional therapy, as per the critical needs of the vulnerable CKDu patients, is of utmost importance in preventing malnutrition, dehydration, and uremic symptoms. There is an unmet need to look at the etiology of CKDu in the South Asian region and tailor the nutritional therapy in the low-resource setting.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol. 2012;13:10.
- [Google Scholar]
- Organochlorine pesticide level in patients with chronic kidney disease of unknown etiology and its association with renal function. Environ Health Prev Med. 2017;22:49.
- [Google Scholar]
- Chronic kidney disease of unknown etiology in agricultural communities. MEDICC Rev. 2014;16:9-15.
- [Google Scholar]
- Dose escalation, safety and impact of a strain-specific probiotic (Renadyl™) on stages III and IV chronic kidney disease patients. J Nephrol Ther. 2013;3:3.
- [Google Scholar]
- The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657-70.
- [Google Scholar]
- p-Cresylsulfate and indoxylsulfate level at different stages of chronic kidney disease. J Clin Lab Anal. 2011;25:191-7.
- [Google Scholar]
- Subclinical vitamin K deficiency in hemodialysispatients. Am J Kidney Dis. 2007;49:432-9.
- [Google Scholar]
- Proceedings: The production of vitamin K by human intestinal bacteria. J Med Microbiol. 1975;8:Pix.
- [Google Scholar]
- Probiotics, prebiotics and synbiotics – A review. J Food Sci Technol. 2015;52:7577-87.
- [Google Scholar]
- p-Cresylsulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant. 2010;25:219-24.
- [Google Scholar]
- High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012;81:300-6.
- [Google Scholar]
- Bacteriocin production by Streptococcus thermophilus in complex growth media. Biotechnol Lett. 2016;38:1947-54.
- [Google Scholar]
- Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol. 1983;45:1808-15.
- [Google Scholar]
- Bacteriocin production by Bifidobacterium spp. A review. Biotechnol Adv. 2013;31:482-8.
- [Google Scholar]
- Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075-84.
- [Google Scholar]
- Protective effect of N-acetylcysteine on progression to end-stage renal disease: Necessity for prospective clinical trial. Eur J Intern Med. 2017;44:67-73.
- [Google Scholar]
- The effect of N-acetylcysteine on blood pressure and markers of cardiovascular risk in non-diabetic patients with chronic kidney disease: A placebo-controlled, randomized, cross-over study. Med Sci Monit. 2010;16:PI13-8.
- [Google Scholar]
- Mitch-Requirements for protein, calories, and Fat in the predialysis patient. Handbook of Nutrition and the Kidney, fifth edition. 2006;6:115-37.
- [Google Scholar]
- Kidney disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: A European Renal Best Practice position statement. Nephrol Dial Transplant. 2013;28:1346-59.
- [Google Scholar]
- Probiotic dietary supplementation in patients with stage III and IV chronic kidney disease. Curr Med Res Opin. 2009;25:1919-30.
- [Google Scholar]
- Effect of probiotic on human blood urea levels in patient with chronic renal failure. Nutr Hosp. 2014;29:582-90.
- [Google Scholar]
- Effect of prebiotic and probiotic supplementation in CKD. Kidney Res Clin Pract. 2012;31:A67.
- [Google Scholar]
- Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr. 1995;125:1401-12.
- [Google Scholar]
- Efficacy of probiotics supplementation on chronic kidney disease: A systematic review and meta-analysis. Kidney Blood Press Res. 2018;43:1623-35.
- [Google Scholar]
- Safety of Probiotics Used to Reduce Risk and Prevent or Treat Disease Rockville, MD: Agency for Healthcare Research and Quality. 2011:200.
- [Google Scholar]







