Translate this page into:
How to Give Dietary Advice to Patients with Kidney Disease?
Corresponding author: Narayan Prasad, Department of Nephrology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India. E-mail: narayan.nephro@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sinha A, Prasad N. How to Give Dietary Advice to Patients with Kidney Disease? Indian J Nephrol. 2025;35:178-86. doi: 10.25259/IJN_139_2024
Abstract
Patients with chronic kidney disease (CKD) display a variety of metabolic and nutritional irregularities, with majority of patients already being malnourished before starting dialysis. The screening, assessment and monitoring of nutritional status using an amalgamation of valid, complementary methods is crucial. Early and suitable dietary intervention is vital for preventing, diagnosing and treating malnutrition. All the misconceptions and myths about diet and food need to be resolved. Patient-centric realistic meal plans and dietary counseling are initiated at the early stages of CKD and the commencement of dialysis, with regular follow-ups on an ongoing basis with diet diaries that help prevent malnutrition. This review article will discuss the practical and simple dietary approaches for counseling patients to increase dietary compliance and meet the recommended requirements.
Keywords
Dietary adherence
Dietary pattern and advice
Kidney disease
Malnutrition
Potassium
Protein
Salt
Introduction
Chronic kidney disease (CKD) is a global health problem affecting 850 million people worldwide.1 One of the most important interventions in slowing down CKD progression is dietary intervention. Studies have reported a malnutrition rate of around 18–75% among CKD patients, which is associated with increased morbidities and mortality.2,3 As CKD advances, the requirements and consumption of various nutrients alter considerably.4 Patients may find it perplexing that dietary advice changes based on the CKD stages.5
Comorbidities complicate the dietary intervention and interfere with CKD diet prescription, making dietary adherence further tricky. Therefore, diet-related communication with patients and relatives must be frequent, simple, and straightforward. Understanding medical nutrition therapy (MNT) thoroughly before implementing it is crucial. Instead of generalized dietary instructions for everyone, a practical, patient-centered meal plan is more appropriate.
Providing alternative judicious food choices tailored to the patient’s likes and dislikes to replace restricted foods is more imperative than focusing on restrictions to improve dietary adherence. Early and suitable nutritional intervention is crucial. There has been a paradigm shift from managing specific nutrients towards the broader outlook of whole dietary patterns in recent decades.4 The dietary guidelines now suggest that specific nutrient restriction is unnecessary unless serum levels are elevated unsafely. It is important to screen and assess patients at least subjectively and, if possible, objectively for nutritional status before recommending diet to provide a nutritional diagnosis as a foundation for nutrition therapy. The general dos and don’ts regarding dietary advice in kidney disease are summarized in Figure 1.
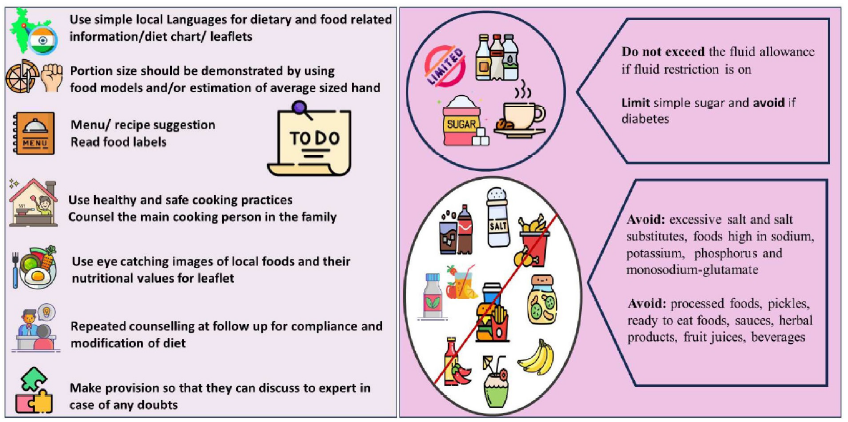
- Do’s and don’ts regarding dietary advice in kidney disease.
Protein intake
A low protein diet (LPD), by reducing hyperfiltration at the glomerular bed, is commonly recommended for slowing the progression of CKD.6 Implementing this advice requires a full understanding of context and dietary habits of the patients. LPD should not be advised in patients with poor appetite and poor nutritional status. Average Indians usually take low (0.6–0.8 g/kg) protein diet daily.6 Avoiding common protein-containing items like pulses and milk without proper dietary advice to meet the daily requirements leads to protein-energy malnutrition.7 The dietary protein requirement in different stages of CKD is shown in Table 1. Customized dietary advice rather than a blanket ban on proteins is required. For stable patients without inter-current illnesses, up to 0.8 g/kg/day in early CKD stage, 1–2 is recommended, which usually can be achieved in the Indian context without recommending any restriction.8 Acute illnesses and infections, prevalent in CKD patients require increasing energy and protein intake independently of the CKD stage.9 It is, therefore, crucial to assess total dietary energy and protein intake before modifying the diet. Vegetarians may be allowed to continue their diet without restricting protein. Patients frequently consuming non-vegetarian meals should be evaluated for protein content in their diet, and dietary changes may be modified as needed. The Indian chronic kidney disease study has shown that even those identifying themselves as non-vegetarian consume meat about once in a week.10 Intake of non-vegetarian foods can be maintained by replacing other protein sources in that meal with non-vegetarian meals in frequency and quantity recommended by dieticians while maintaining nutrient balance. Patients should be encouraged to keep a diet diary to assess dietary adherence.
| CKD stage | Protein requirement | Remarks |
|---|---|---|
| Early CKD stage 1–2 | 0.8 g/kg/day | Stable patients without inter-current illnesses. |
| ND-CKD stage 3–5 | 0.8 g/kg/day | For metabolically stable patients, not on dialysis. Reduce the risk of ESKD and improve QoL. |
| Diabetic-CKD stage 3-5 | 0.8 g/kg/day | Patient not on dialysis. Optimize glycemic control. |
| Very low protein diet (VLPD) stage 3–5 | 0.3–0.4 (average 0.3) g/kg body weight/day | Ketoanalogue supplementation (0.6 g/kg/day) is generally required with VLPD prescription to prevent nitrogen imbalance and malnutrition in CKD. |
CKD: Chronic kidney disease, ND-CKD: Non-diabetic chronic kidney disease, QoL: Quality of life. Supplementing EAAs and their KAs allows prescribing a protein intake below the minimum daily requirement (0.6 g/kg/day) to meet the requirements with the supplements. ESKD: end stage kidney disease, EAA: essential amino acids, KA: ketoanalogues
Very low protein diet (VLPD) with ketoanalogue (KA) supplementation
Kidney disease outcome quality initiatives (KDOQI)11 suggested using a very low protein diet (VLPD) supplemented with ketoanalogues (KAs) to retard the progression of CKD. Implementation of this advice is usually impractrical in Indian setting, mainly because of limited experienced dieticians with hands-on training for such dietary intervention.12,13 A meta-analysis of 10 small randomized control trials (RCTs) and 2 non-RCTs (n = 951) concluded that VLPD supplemented with KAs could slow down the progression of CKD patients with eGFR > 18 mL/min/1.73 m2 as compared to placebo; however, there was no benefit when compared to an active comparator with patients on low protein diet.14 The high prevalence of malnutrition and lower daily protein intake in Indians make it further inadvisable to prescribe VLPD. Moreover, the advocated dose of 1 tablet/5 kg body weight of ketoanalogs adds significant pill burden besides being expensive. Its use may be considered properly selected patients with slowly progressive kidney diseases with good appetite and normal nutritional status who can tolerate and afford the costly ketoanalogues, under the constant supervision of a skilled and experienced dietician and physician.14
Type of protein
The qualitative aspects of food (plant or animal, fresh or preserved, and culinary methods) are also important.15 There is uncertainty about whether the source of protein, plant and animal, impacts CKD progression differently.5 The shift from animal protein-based to plant-based diets is being increasingly recommended for its pleotropc benefits on cardiovascular health. Plant-based diets could has a favorable effect on gut microbiota, and may be better for control of nitrogen balance, acid–base metabolism, and bone mineral disorders. Vegetarian diets generate fewer uremic toxins, help with reduction of salt intake, and reduce saturated fat intake.
Plant proteins are acid-neutral or alkali-producing, while animal proteins upsurge dietary acid load in kidney disease.16 Animal proteins may be associated with higher glomerular hyperfiltration than plant proteins.17 Plant-based meal with comparable nutrients resulted in reduced levels of serum phosphate, urine phosphate excretion, and fibroblast growth factor-23.18
A plant-based diet with animal foods like egg whites, low-fat milk and milk derivatives, and lean meat will often meet nutrient needs. Based on patients’ taste for animal or plant protein, dieticians must ensure that they achieve their energy protein requirements and have adequate essential amino acids (EAA) through palatable recipes. Updated recommendations support prescribing adequate fruit and vegetables in non-dialysis-dependent-CKD (NDD-CKD) stages 1 to 4 to reduce weight, blood pressure, and net acid production.11
Dietary fat
Patients typically develop dyslipidemia when GFR begins to decline.4 American Heart Association (AHA) advises a total fat intake of 25–35% of total calories, monounsaturated fatty acid (MUFA) up to 20%, and polyunsaturated fatty acids (PUFA) up to 10%, SFA not exceeding 7%, trans fats at <1% of total calorie intake.19 Patients with high serum cholesterol should restrict cholesterol to less than 200 mg/day.20 A judicious amalgamation of cereals, pulses, vegetables, milk, and a variety of vegetable oils aids in maintaining optimal fatty acid composition. Minimization of reusing and reheating of oil is also important. The practice of rotating oils should be encouraged. The recommended combination of oils (groundnut or sesame or rice bran + mustard oil/canola oil/soybean oil) suggested by the National Institute of Nutrition (NIN) may be useful as it helps to maintain a balance to give all the essential nutrients and also maintain the desirable omega – 6 to omega 3 – ratios.18
Energy intake
The KDOQI guidelines 2020 recommend an energy intake of 25–35 kcal/kg ideal body weight in CKD patients based on age, gender, physical activity, weight, CKD stage, comorbidity, and inflammation to maintain nutritional status.11 CKD patients must not be energy deficient. Our study revealed that the calorie intake of CKD patients on initiation of dialysis is less than the recommended levels.21 An often unintended undesirable effect of dietary advice is severe reduction in calorie intake which further contributes to malnutrition.
Carbohydrate intake
When protein is limited, carbohydrate consumption must be sufficient to meet energy needs, prevent starvation ketosis, spare protein, and provide fibers. Mixed cereals like rice and rice derivatives – poha, aliya, etc., wheat flour, porridge, stevia, suji upma, suji chila, suji idli, daal sago, etc. can be used. Cereals should be used with pulses, peas, milk, and milk products to mutually complement proteins and improve protein quality. Small frequent meals may be advised. Sago (low protein, negligible phosphorus) can be used in the diet in various recipes adjusted for salt/sugar amount. Arrowroot/sago/rice flour can be blended with wheat flour if it suits the patient’s taste. Desserts can be prepared with sago, rice, and arrowroot to increase calorie intake and make the diet palatable.
Glycemic control in diabetic patients
For diabetics, timing, quality, and quantity of carbohydrates is crucial. A consistent amount of carbohydrates should be consumed in each meal for patients on fixed doses of insulin or oral hypoglycemic agents with regular meal timings. Patients need to be educated about food exchange lists, carbohydrate counting, and experience-based portion size estimation. Teaching patients to read food labels and use food pyramids, measuring cups, spoons, and healthy food plates helps them choose the proper amount and quality of food. Educating them regarding glycemic index and glycemic load may be beneficial for sugar control.
Fiber
CKD patients should not be deprived of the advantages of fiber in their diet, which also helps in preventing constipation. A daily fiber intake of 25–30 g/day or more for CKD patients may be suggested.22 When consumed in moderation as part of a healthy diet, naturally occurring sugars in fruit, vegetables, milk, and curd do not cause health hazards associated with free sugars.4
Electrolytes balance
Electrolyte imbalance occurs in all stages of CKD, predominantly when GFR falls very low. A patient-friendly nutrition blend of phosphorus, potassium, and sodium aids in dietary compliance.
Dietary potassium
Hyperkalemia develops as GFR declines. Dietary modifications are now recommended only to treat hyperkalemia and not as a preventative measure. KDOQI 2020 guidelines recommend adjusting dietary potassium intake to maintain normal serum potassium.4 Causes of hyperkalemia need to be identified and rectified after taking patients’ history and reviewing the medicine prescription. One must also check if patients are taking low-sodium salt substitutes, which are usually high in potassium. The sodium and potassium content of common edible salt are shown in Table 2. Patients may over-restrict fruit, vegetables, legumes, and whole grains as food composition tables do not take into account bioavailability, culinary practices, portion sizes, food combinations, fiber, or diabetic state.23
| Type of salt | Na | K |
|---|---|---|
| Tata common salt | 38 g | |
| Tata salt (pink salt) | 38 g | - |
| Tata rock salt (premium Sendha Namak) | 37.4 g | 10 mg |
| Tata salt lite (green pack) | 33.2 g | 7.8 g |
| Tata salt super lite (30% less Na iodised salt) | 26.85 g | 15.75 mg |
| Catch sprinklers, black salt | 38.79 g | |
| Catch sprinkler table salt | 33.5 g | 2.5 mg |
| Himalaya pink salt | 37.4 g | 10 mg |
| Himalaya pink salt (by Nature) | 33.83 g | 9.18 mg |
| PRO NATURE Classic rock salt (Sendha Namak) | 38.79 g | 0.69 g |
| Saindhavalavana powder rock salt | 38 g | 160 mg |
| WeiKFiELD baking powder | 12 g | |
| WeiKFiELD baking soda | 26 g | |
| Ashoka baking soda | 22.94 g | |
| Azinomoto | 125 mg/1/4th tsp | |
| Organic platter ajinomoto chinese salt, MSG | 30 mg |
Source: As per the ingredient information printed on the product. Na: Sodium, K: Potassium, MSG: Monosodium glutamate
Personalized dietary advice is a cornerstone in the management of CKD patients. Figure 2 shows the flowchart for individualized dietary prescriptions for CKD to maintain or improve nutritional status and slowing disease progression. To judiciously plan a diet for a CKD patient, first, consider energy and protein intake and select low phosphorus sources of protein. After deciding on the aforementioned, milk and milk products, eggs, cereals, and pulses are set; the amount of fruits and vegetables can be decided based on potassium needs. Potassium sources (fruits, vegetables, legumes, and nuts) are also abundant in fiber and other micronutrients. It is suggested to avoid routinely restricting them unless the serum potassium level is high and non-dietary causes of hyperkalemia have been addressed.4 Leaching of vegetables is only required for patients with hyperkalemia. Patients should be advised to choose low-potassium fruits and vegetables in case of hyperkalemia. Salt substitutes rather than vegetables and fruits are the culprits in most cases. Many patients use rock salt, low sodium salt, etc, which are high in potassium [Table 2].
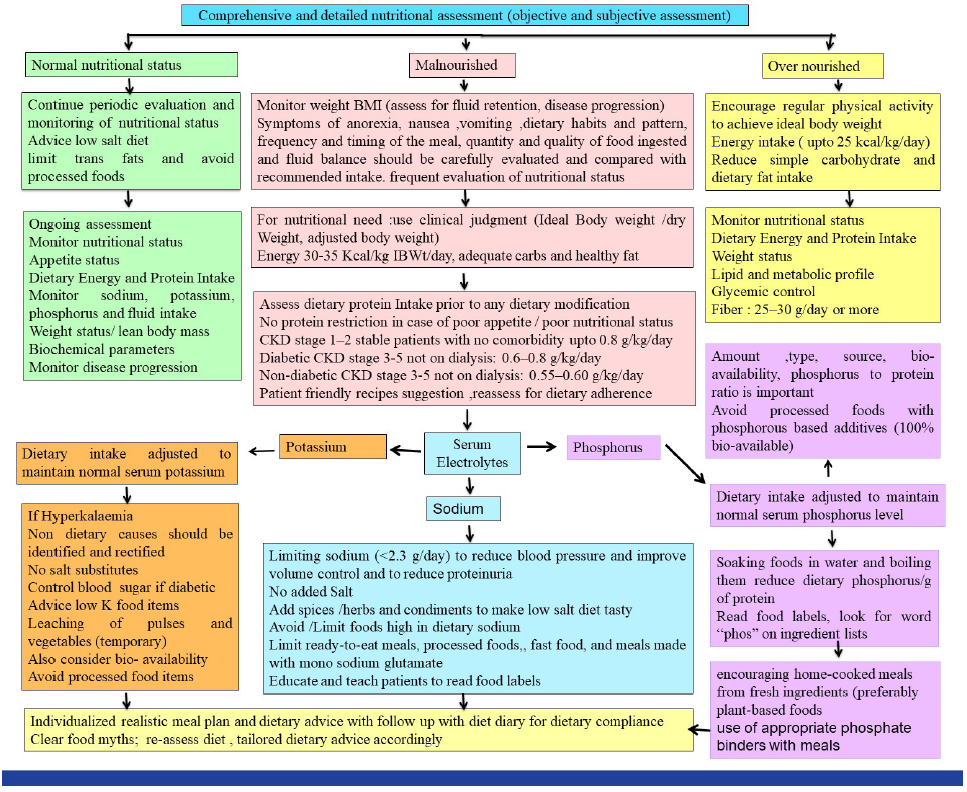
- Tailored dietary advice in chronic kidney disease (CKD).
Patients should be educated regarding the potassium content of common Indian foods, as shown in Table 3, calculated based on the Indian Food Composition Tables (IFCT) 2017 database.24 Renal-specific cut-off points for dietary potassium have been shown in Table 3.25
| Food items |
Potassium (mEq) 1 mg = 39 mEq |
Renal-specific cut-off points for dietary potassium mg/100 g25 |
|---|---|---|
|
1 Roti (25 g) refined wheat flour 1 Roti (25 g) whole wheat flour |
∼1 mEq ∼2 mEq |
Low <100 mg/100 g Medium : 101–200 mg High 201–300 mg Very high >300 mg Low means desirable, Medium means moderately desirable, High means not desirable and very high means detrimental |
| 1 Bowl of dal (25–30g) | 9–10 mEq | |
| Green leafy vegetables | 10–15 mEq | |
|
Milk cow 250 mL Milk buffalo 250 mL |
7.37 mEq 6.98 mEq |
|
| Rice/rice flakes/puffed rice (25 g) | 0.9–0.95 mEq | |
| *Tata sodium lite salt (5 g) | 10 mEq | |
| Tomato ripe (50 g) | 2.62 mEq | |
| Coconut water (100 mL) | 5.5 mEq | |
| Bel fruit (100 g) | 10.49 mEq | |
| Turmeric (2.5 g) | 1.52 mEq | |
| Jeera (2.5 g) | 1.20 mEq | |
| Hing (1 g) | 0.06 mEq | |
| Methi seeds (2.5 g) | 0.57 mEq | |
| Laung (1 g) | 0.37 mEq | |
| Spinach (100 g) | 16.03 mEq | |
| Bathua (100 g) | 11.23 mEq | |
| Chaulai (100 g) | 14.66 mEq | |
| Methi saag (100 g) | 5.79 mEq | |
| Lemon juice (10 mL) | 0.29 mEq | |
| Potato, brown skin, big (100 g) | 13.87 mEq | |
|
Potato, brown skin, small (100 g) |
12.15 mEq |
Bel fruit, green leafy vegetables, coconut water, fruit juice, vegetable soups, and salt substitutes are high in potassium and should be avoided in patients with advanced kidney disease. Their routine restriction in mild-moderate CKD is not advisable given the beneficial effect of dietary potassium on blood pressure. Spices and condiments can be used after quantifying their potassium content and controlling portion size. Garlic, heeng, methi dana, and turmeric can enhance the flavor and palatability of meals with limited salt. Use coriander powder (dhania), cumin seeds (jeera), and red chili powder in small quantities (just to change the taste). Dietary calculations are based on raw values. We really need Indian data regarding the bioavailability of potassium in foods and robust data about potassium losses during washing and cooking to be aware of the raw-to-cooked ratio.
Bioavailability of potassium
Patients and physicians should also be aware that plant-based potassium is less bioavailable than animal foods and food additives.26 Hidden potassium sources include ultra-processed foods containing potassium additives, salt substitutes, and potassium preservatives. Potassium additives are almost 100% bioavailable.27 Potassium intake from additives and animal sources should be reduced due to high bioavailability.
Phosphorus intake
Dietary phosphorus restriction is challenging, especially for those on dialysis needing a high-protein diet. Dietary phosphorus intake should be adjusted to maintain serum phosphorus levels in the normal range.11
It is vital to consider the amount of dietary phosphorus, its type (organic vs. inorganic), source (animal vs. plant), protein-to-phosphorus ratio, and bioavailability.28 Dietary education helps to reduce phosphorus intake while keeping adequate protein intake. Boiling reduces phosphorus, sodium, and potassium content in the food.29
Animal and plant proteins contain organic phosphorus. Animal proteins have higher phosphorus bioavailability (40%–60%) than plant proteins (20%–40%) due to the presence of phytates, which are not readily available. The low bioavailability of plant-based phosphorus helps in planning a diet limited in phosphorus with adequate protein for dialysis patients.
Beverages like colas, enhanced meats, frozen meals, processed or spreadable cheeses, instant foods, and refrigerated bakery products are rich in inorganic phosphorus > 90 % of which is bio-available. Food additives have high bioavailability (∼100%).30 Patients should develop a habit of looking for the word “phos” on ingredient lists to be aware of hidden phosphorus sources.
The dietary phosphorus/protein ratio aids in controlling high-phosphorus intake while ensuring adequate protein intake. The phosphorus-to-protein ratio of common Indian foods is given in Table 4. Renal-specific nutrient cut-off points for phosphorus and phosphorus-to-protein ratio are mentioned in Table 4.25 Egg whites have the lowest phosphorus-to-protein ratio, which is less than 2:1. The phosphorus content of the whole pulse is more or less similar to the washed pulse or even lower [Table 4]. Therefore, whole pulses should not be restricted as suggested in routine for kidney patients. Restricting non-protein sources of phosphorus is useful in preventing malnutrition. Soaking foods in water and boiling them can help reduce dietary phosphorus/gram of protein.
| Food items |
Phosphorus to protein ratio |
Renal-specific cut off points for phosphorus to protein ratio (mg/g)25 |
|---|---|---|
| Wheat flour whole | 30.29 |
Low < 12 mg/g Medium 12–15 mg/g High >15 mg/g low means desirable, medium means moderately desirable, high means not desirable and very high means detrimental |
| Wheat flour refined | 10.62 | |
| Milk buffalo’s | 23.6 | |
| Milk cow’s | 29.24 | |
| Paneer | 17.46 | |
| All poultry, Egg (whole) | 15.59 | |
| Egg white | 1.86 | |
| Egg yolk | 36.6 | |
| Rice, raw, brown | 29.15 | |
| Rice, parboiled, milled | 17.95 | |
| Rice, raw, milled | 12.12 | |
| Raw dals (average) | 16.7 | |
| Goat, legs | 8.47 | |
| Goat, kidney | 14.1 | |
| Goat, liver | 16.53 | |
| Fishes (fresh water) Crab (40 g) | 15.72 | |
| Rohu (40 g) | 10.15 | |
| Prawn, big (40 g) | 16.42 | |
| Prawn, small (40 g) | 15.07 | |
| Chicken, leg, skinless | 10.24 | |
| Chicken, thigh, skinless | 10.23 | |
| Chicken, breast, skinless | 8.165 | |
| Chicken, wing, skinless | 10.86 | |
| Chicken liver | 12.56 |
IFCT: Indian food composition tables, NIN: National institute of nutrition, ICMR: Indian council of medical research.
Dietary sodium and salt
A low salt diet is a cornerstone in CKD management, except in salt-wasting kidney diseases.28 Guidelines recommend a sodium intake of <2.3 g/d.11 A systematic review reported a high salt intake of 11 g/day in the general population in India,31 which is much higher than recommended. Similarly, the Chronic Renal Insufficiency Cohort (CRIC) study reported that only about 25% of CKD patients had a sodium intake <100 mmol/24 hours, assessed by three readings.32 Reducing salt intake is crucial from the early stages to the end stages of kidney disease.
Practical tips to reduce salt and sodium in daily diet: Gradually cutting back on salt over a few weeks is crucial when cooking and preparing meals. Meals must be made using fresh ingredients and either cooked without salt initially, then finished with the recommended amount later, or without salt and added the prescribed amount afterward. Pickles, papad, chutney, sauces, salted butter and nuts, ready-to-eat meals, processed foods, canned foods, fastfood, and meals made with monosodium glutamate should be limited or avoided. Spices, herbs, condiments like ginger, garlic, onion, kokum, tamarind, lemon, grated mangos, vinegar, bay leaf, cinnamon, cloves, nutmeg, cumin, and black pepper can be used to make a low-salt diet palatable, keeping portion size in mind. Patients must be trained to read food packaging’s nutrition facts labels.
Fluid management
Urine output is usually normal in CKD stages 1 to 3. Fluid intake of 1.5–2 L/d may be prescribed except in edematous patients.33 Fluid recommendations, however, vary based on individual weight gain (IDWG), blood pressure, and residual renal functions and a customized strategy is required. Teaching patients to manage fluid intake and recognize symptoms of fluid overload is advocated. A limited intake of salty and fried foods to quench thirst is advised. Patients can use paneer or hung curd instead of milk and use thick dal/gravy instead of watery pulse. It is suggested that allowances be distributed throughout the day, using small glasses or cups, medication with mealtime beverages, and not with extra water.
Modification in dietary advice in kidney diseases other than CKD
Comparative changes in the nutritional requirement in nephrotic syndrome, dialysis and transplant patients are summarized in Table 5, and AKI is given in Table 6.
Nephrotic syndrome
The dietary goal in nephrotic syndrome is to reduce the associated symptoms like edema and dyslipidemia, compensate for urinary albumin losses, spare dietary proteins, and slow the progression of kidney disease. Individualized dietary advice is important in the acute phase as well as during remission. Low salt with a “no added salt diet” is advised to minimize edema and hypertension. Fluid and sodium balance is vital. The salt and fluid restriction is usually required only until the patient achieves remission. Simple sugars should be limited, and complex carbohydrates should be preferred. Fat intake should be low.
Acute kidney injury (AKI)
The dietary management of malnourished, hypercatabolic AKI patients is challenging. Non-catabolic patients with recovering renal function and most on continuous renal replacement therapy (CRRT) need a dietary protein intake of around 0.8 g/kg/day however, critically sick AKI patients needing CRRT will require a much higher protein intake (1.5 g/kg/day with an additional 0.2 g/kg/day to compensate for amino acid/protein loss during renal replacement therapy).34,35 Timely and suitable initiation of enteral and parenteral nutrition is essential as per requirement.
| Dialysis11 | Transplantation11,36,37 | Nephrotic syndrome | |
|---|---|---|---|
| Energy (kcal/kg ideal weight/day) | 25–35 kcal/kg/day |
25–35 kcal/kg/day in maintenance kidney transplant patients 25 kcal/kg/day (obesity) 35–40 kcal/kg/day for the first 4 weeks after transplantation |
30–35 kcal/kg/day Calorie restriction may be needed in overweight or obese patients |
| Protein (g/kg/day) | 1.0–1.2 g/kg body weight |
0.8 (CKD stages 3–5 T) ≥1.4 (for the first 4 weeks after transplantation or if high doses of prednisone are required |
0.8 g/kg/day One additional gram of high biological value dietary protein for each gram of urinary loss is needed. |
| Sodium (g/day) | <2.3 g/day | <2.3 g/day | 1–3 g/day sodium to control edema |
| Potassium (mg/day) | Adjust dietary potassium intake to maintain serum potassium level | Adjust dietary potassium intake to maintain serum potassium within the normal range | |
| Phosphorus (mg/day) | Adjusting dietary phosphorus intake to maintain serum phosphate levels in the normal range | Adjust dietary phosphorus intake to maintain serum phosphate levels in the normal range |
CKD: Chronic kidney disease
| Energy | AKI |
|---|---|
| Non-protein calories (kcal) | 25 kcal/kg/day |
| Carbohydrates (g) | 5 g/kg/day |
| Fat (g) | 0.8–1.2 g/kg/day |
| Protein (essential and non-essential amino acids) | |
| Conservative therapy, mild catabolism | 0.8 g/kg/day |
| Extracorporeal therapy, moderate catabolism | 1.0–1.5 g/kg/day |
| CRRT or SLED, severe hypercatabolism | 1.5–2.0 g/kg/day |
CRRT: Continuous renal replacement therapy, SLED: Sustained low-efficiency dialysis, AKI: Acute kidney injury.
Dialysis
Dietary advice changes from low protein to high protein diet to compensate for the losses during dialysis.11 Adequate nutritional support and dietary advice should be provided for adequate energy and protein intake in dialysis patients to maintain or improve their nutritional status.
Renal transplantation
The dietary needs of renal transplant patients change at each phase of transplantation. An adequate diet is important to avoid a negative nitrogen balance. They require adequate calories (35–40 kcal/kg/day) and protein (up to 1.4g/kg/day) for at least 4 weeks for wound healing, fast recovery, fighting infection, preventing protein catabolism, preserving muscle mass, and managing acute complications.36,37 Adequate glycemic control is very important. In the maintenance phase, energy is slightly decreased. Reduced calorie intake is needed in obese patients on follow-up.36 Intake of dietary potassium and phosphorus is adjusted to maintain serum values within the normal range.11 Food safety and hygiene are crucial.
Common myths regarding dietary restrictions among kidney patients
Many patients use salt substitutes like low sodium salts or rock salt (sendha namak), which are high in potassium and may lead to hyperkalemia. Many patients use pomegranate/orange/sweet lime/carrot/beetroot juice (high in potassium) to increase hemoglobin as they are unaware of renal anemia. A common myth is to avoid all protein sources like pulses, milk and milk products, which leads to protein malnutrition. The key is to consume the right quantity and quality of dietary protein as per advice from a qualified dietician. A dietary amino acid (AA) pattern with a higher load of a branched-chain, alcoholic, and aromatic AAs may increase the risk of CKD. However, dietary AA pattern rich in acidic AAs and proline and a lower load of alkaline AAs and small AAs could decrease the risk of CKD. However, it needs to to confirmed in a larger study.38
Another food myth is that kidney patients should avoid fruits and restrict vegetable intake or do leaching of all pulses and vegetables. All vegetables do not need leaching as it leads to the loss of water-soluble vitamins and minerals. Leaching is required only for hyperkalemic patients. Fruits and vegetables are also rich in fibers, vitamins, and phytochemicals. All CKD patients do not require a low-potassium diet.
Regarding fluid restrictions, most patients understand that only water intake is restricted; however, they need to be counseled about other liquids, including liquid in food. Some patients believe that they should drink a large amount of water to increase urine output. They need to be counseled about how fluid allowances are individualized. Patients with renal calculi and urinary tract infections having normal kidney function are advised to consume plenty of fluids. In early CKD diseases with good urine output and without any evidences of fluid overload, water intake may be liberal to quench the thirst. However, with evidences of fluid overload, it needs to be restricted.
Dietary myths, if not clarified, worsen the diet quality, leading to compromised nutritional intake and nutritional status in these patients. Dietary education programs should be conducted involving patients and their family members/caregivers from time to time to enhance their nutrition knowledge and dietary practices.
Nutritional recommendations for renal disease are typically restrictive, so suitable dietary advice with ongoing nutrition education is essential. Taking a detailed diet history is of utmost importance for proper diet planning. Food myths should be dispelled using dietary teaching tools like ready reckoner or pamphlets or leave-behind hand-outs in the local language with eye-catching pictures. Individualized and practical dietary advice should begin in the early stages of CKD, at the start of dialysis, and at every follow-up with diet diaries. A holistic nutritional strategy is crucial, rather than focusing on individual nutrient restriction.
Conflicts of interest
There are no conflicts of interest.
References
- A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019;96:1048-50.
- [CrossRef] [PubMed] [Google Scholar]
- McCollum award lecture, 1996: Protein-energy malnutrition in maintenance dialysis patients. Am J Clin Nutr. 1997;65:1544-57.
- [CrossRef] [PubMed] [Google Scholar]
- Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am J Kidney Dis. 2003;42:864-81.
- [CrossRef] [PubMed] [Google Scholar]
- Nutrition in kidney disease: Core curriculum 2022. Am J Kidney Dis. 2022;79:437-49.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease: Role of diet for a reduction in the severity of the disease. Nutrients. 2021;13:3277.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of low protein diet in management of different stages of chronic kidney disease - practical aspects. BMC Nephrol. 2016;17:156.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18:254-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Low-protein diets for chronic kidney disease patients: The Italian experience. BMC Nephrol. 2016;17:77.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clinical Nutrition. 2021;40:1644-68.
- [CrossRef] [PubMed] [Google Scholar]
- The Indian Chronic Kidney Disease (ICKD) study: Baseline characteristics. Clin. Kidney J. 2022;15:60-9. doi: https://doi.org/10.1093/ckj/sfab149
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76:S1-107.
- [CrossRef] [PubMed] [Google Scholar]
- Very low-protein diets in advanced kidney disease: Safe, effective, but not practical. Am J Clin Nutr. 2022;115:1266-7.
- [CrossRef] [PubMed] [Google Scholar]
- The international society of renal nutrition and metabolism commentary on the national kidney foundation and academy of nutrition and dietetics KDOQI clinical practice guideline for nutrition in chronic kidney disease. J Ren Nutr. 2021;31:116-20.e1.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effect of ketoanalogues on chronic kidney disease deterioration: A meta-analysis. Nutrients. 2019;11:957.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016;17:102.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Plant-based diets for kidney disease: A guide for clinicians. Am J Kidney Dis. 2021;77:287-96.
- [CrossRef] [PubMed] [Google Scholar]
- Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990;38:136-44.
- [CrossRef] [PubMed] [Google Scholar]
- Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dietary fat and heart health: In search of the ideal fat. Asia Pac J Clin Nutr. 2002;11 Suppl 7:S394-400.
- [CrossRef] [PubMed] [Google Scholar]
- Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486-97.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in nutritional status on follow-up of an incident cohort of continuous ambulatory peritoneal dialysis patients. J Ren Nutr. 2008;18:195-201.
- [CrossRef] [PubMed] [Google Scholar]
- Retarding chronic kidney disease (CKD) progression: A practical nutritional approach for non-dialysis CKD. Nephrology @ Point of Care. 2016;2:pocj.5000207.
- [CrossRef] [Google Scholar]
- Re-thinking hyperkalaemia management in chronic kidney disease—Beyond food tables and nutrition myths: An evidence-based practice review. Nutrients. 2023;16:3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Indian food composition tables. Hyderabad, Telangana State, India: National institute of nutrition, Indian council of medical research; 2017.
- NKF K/DOQI nutrition in chronic renal failure adult guidelines electronic version. Am J Kidney Dis. 2000;35:1-141.
- [CrossRef] [PubMed] [Google Scholar]
- An investigation into the bioaccessibility of potassium in unprocessed fruits and vegetables. Int J Food Sci Nutr. 2008;59:438-50.
- [CrossRef] [PubMed] [Google Scholar]
- Potassium additives and bioavailability: Are we missing something in hyperkalemia management? J Ren Nutr. 2019;29:350-3.
- [CrossRef] [PubMed] [Google Scholar]
- Importance of renal dietitians in nutritional counselling and dietary interventions in the early stages of chronic kidney disease. J Nephro & Endo Res 2023:1-5.
- [PubMed] [Google Scholar]
- Management of natural and added dietary phosphorus burden in kidney disease. Semin Nephrol. 2013;33:180-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Phytate and kidney health: The roles of dietary phytate in inhibiting intestinal phosphorus absorption and intravenous phytate in decreasing soft tissue calcification. J Ren Nutr. 2023;33:225-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mean population salt consumption in India: A systematic review. J Hypertens. 2017;35:3-9.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. 2016;27:1202-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Electrolyte and acid-base balance disorders in advanced chronic kidney disease. Nefrologia. 2008;28(Suppl 3):87-93.
- [Google Scholar]
- Dietary management in acute kidney injury. Clinical Queries: Nephrology. 2012;1:58-69.
- [CrossRef] [Google Scholar]
- Protein requirement in adult kidney transplant recipients. Nephrology.. 2010;15(Suppl 1):S68-71.
- [CrossRef] [Google Scholar]
- Dietary amino acid patterns are associated with incidence of chronic kidney disease. J Ren Nutr.. 2022;32:312-318.
- [CrossRef] [PubMed] [Google Scholar]







