Translate this page into:
Humoral Immune Response to BBV152 (Covaxin) SARS-CoV-2 Vaccine in Patients on Hemodialysis
Address for correspondence: Dr. Ramasubramanian Viswanathan, Department of Nephrology, Multispecialty Hospital, Tirunelveli Medical College Hospital, Tirunelveli - 627 011, Tamil Nadu, India. E-mail: nephpubtvmch@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Dear Editor,
Chronic kidney disease (CKD) patients on maintenance hemodialysis (HD) have increased morbidity and mortality with coronavirus disease 2019 (COVID-19) infection and are prioritized to be vaccinated. BBV152-inactivated vaccine Covaxin was developed in India and approved by the Drugs Controller General of India for emergency use in January 2021 for adults ≥18 years of age. A Phase 2 trial indicated a seroconversion rate of 88%–98.3% in healthy volunteers.[1] There is paucity of data on the efficacy of Covaxin in HD patients. This study was undertaken to analyze the humoral immune response to BBV152 in HD patients at a tertiary government hospital in South India.
This is a cross-sectional, observational study conducted from September 2021 to February 2022. Ethics committee approval was obtained with EC registration ID ECR/1227/Inst/TN/2019, protocol number 20212238. All CKD stage 5 patients ≥18 years of age on maintenance HD and who gave informed consent to take BBV152 were included in the study. Patients with age <18 years, patients with intercurrent illness, those with previous polymerase chain reaction (PCR)-confirmed diagnosis of COVID-19, and those with baseline positive antibody titers were excluded. Demographic, clinical, and laboratory parameters were collected as per a structured questionnaire.
Blood samples were drawn at baseline and 4 weeks after each dose of vaccine administration. IgG antibody testing was done by Access 2 Immunoassay System – Beckman Coulter, a qualitative test for detecting neutralizing antibodies against the receptor binding domain of spike protein with a sensitivity of 96.8% and specificity of 99.6%. Result was reported as signal/cutoff ratio (S/CO) ≤0.8 nonreactive and S/CO ≥1 reactive. Data was entered in MS Excel, and analysis was conducted using the Statistical Package for the Social Sciences (SPSS) v26.0. Mean and median were calculated for continuous variables of interest. Normality of the continuous variables was tested by Kolmogorov–Smirnov test and was found to be non-normally distributed. Mann–Whitney test was used to test the significance of difference between the seropositive and seronegative patients. Significance of association between seropositivity and the categorical variables was tested by Chi-square test or Fisher’s exact test. A P value of <0.05 was considered statistically significant.
One hundred and nineteen patients were initially assessed for eligibility, out of which 107 completed the study. Three patients developed intercurrent illness, four developed COVID-19, three patients had baseline high antibody titers, and two defaulted on vaccine schedule. Of the four patients who developed breakthrough infection, three developed it after the first dose and one developed it after the second dose. Mean age of patients was 39.8 years, and 69.2% were males. Also, 86% of patients had dialysis vintage of <24 months. Cause of CKD was diabetic kidney disease in 20.6%, chronic glomerulonephritis in 9.3%, unknown etiology in 54.2%, and the rest (15.9%) comprising chronic interstitial nephritis, congenital anomalies of kidney and urinary tract, obstructive nephropathy, autosomal dominant polycystic kidney disease, systemic lupus erythematosus, and posttransplant graft failure.
Eighty-one out of 107 (75.7%) patients achieved seroconversion after two doses of BBV152 [Figure 1], while 66.4% achieved it after the first dose. Also, 89% of patients reported mild–moderate fever, myalgia, nausea, and injection site pain. No serious adverse events were noted. A study by Giot et al.[2] on 65 HD patients who completed BNT162b2 vaccination showed seropositivity of 77.1%, similar to this study. Yadav et al.[3] from North India showed a seropositivity rate of 88%, while another study from Madurai in South India[4] showed a seroconversion rate of 88.9% after two doses of ChAdOx1-S vaccine. Patients with higher age, diabetes, Atherosclerotic Cardio Vascular Disease (ASCVD), anemia, higher Erythrocyte Sedimentation Rate (ESR), and spKt/V <1.2 had lower seroconversion, while higher serum albumin correlated with seropositivity [Table 1]. Several studies[345] have also observed similar correlation.
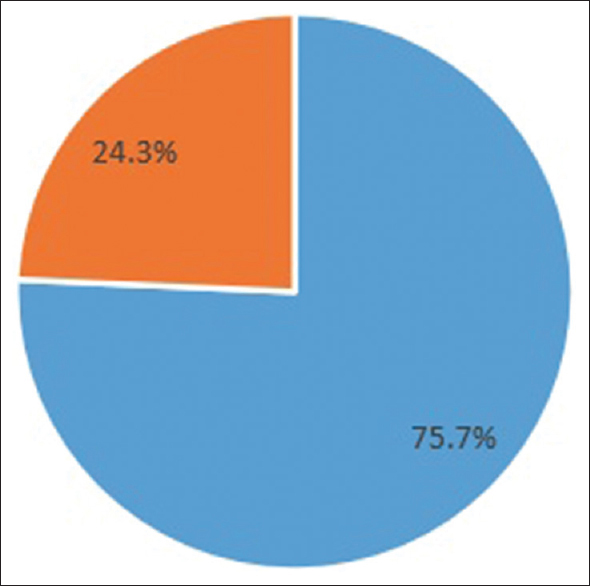
- Seroconversion.
 Seropositive,
Seropositive,  Seronegative
Seronegative| Variable Significance of association (original) | Seropositive (%) | Seronegative (%) | P |
|---|---|---|---|
| Duration of dialysis | |||
| >24 months | 14 (93.3) | 1 (6.7) | 0.07* |
| ≤24 months | 67 (72.8) | 25 (27.2) | |
| Comorbidities | |||
| Diabetes mellitus | 3 (13.6) | 19 (86.4) | <0.001* |
| Hypertension | 70 (72.9) | 26 (27.1) | 0.06 |
| Atherosclerotic cardiovascular disease | 6 (33.3) | 12 (66.7) | <0.001 |
| Chronic obstructive pulmonary disease | 1 (33.3) | 2 (66.7) | 0.15* |
| Chronic liver disease | 0 (0) | 1 (100) | 0.24* |
| Use of immunosuppressant | 3 (75) | 1 (25) | 1.0* |
| Lymphocyte count (<1000) | 10 (66.7) | 5 (33.3) | 0.52 |
| Severe anemia | 50 (68.5) | 23 (31.5) | 0.014 |
| Kt/V | |||
| Adequate dialysis | 76 (83.5) | 15 (16.5) | <0.001 |
| Inadequate dialysis | 5 (31.3) | 11 (68.7) | |
| Significance of variables (original) | Median (IQR) | Median (IQR) | |
| Age | 38 (26-45.5) | 51 (42.8-54.5) | <0.001 |
| Erythrocyte sedimentation rate | 25 (17.5-40) | 47.5 (43.8-55) | <0.001 |
| Serum creatinine | 7.8 (6.2-9.5) | 8.2 (6.9-9.3) | 0.794 |
| Serum albumin | 3.4 (3.1-3.9) | 3 (2.7-3.2) | <0.001 |
IQR=interquartile range P<0.05 is indicated in bold. *Fisher’s exact test Since one of the cells in the contingency table has a frequency of <5
Strength of the study is in excluding patients with previous COVID-19 infection and use of indigenously developed vaccine. During the study period, the predominant strains of COVID-19 were omicron and delta strains and the vaccine efficacy against the different strains needs to be considered. Limitation of the study was its inability to rule out plausible contributory role of memory B cells, difficulty in assessing real-world vaccine protection, and the study being applicable only to Indian ethnicity.
To conclude, this study shows a lower seroconversion rate at 75.7% after two doses of Covaxin. Increasing age, presence of diabetes, ASCVD, anemia, inadequate HD, and hypoalbuminemia were associated with a lower seroconversion. Studying the antibody response as well as T-cell–mediated immunity after 6 months of vaccination and analyzing associated variables would help in formulating further protective doses in this high-risk population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank the Department of Health Research and Multi Disciplinary Unit of ICMR at Tirunelveli Medical College Hospital for assistance in performance of SARS CoV2 IgG antibody testing.
Bibliography
- Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21:950-61.
- [Google Scholar]
- Clin Kidney J. 2021;14:2239-45.
- Humoral response to one and two doses of ChAdOx1-S vaccine in patients on hemodialysis. Clin J Am Soc Nephrol. 2021;16:1875-6.
- [Google Scholar]
- Humoral response to viral vector COVID-19 vaccine in hemodialysis patients. Kidney Res Clin Pract. 2022;41:342-50.
- [Google Scholar]
- Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32:2153-8.
- [Google Scholar]






