Translate this page into:
IgA Nephropathy – Beyond the Renal Cortex
Address for correspondence: Dr. Balaji Kirushnan, Department of Nephrology, Kauvery Hospital, 199, Luz Church Road, Alwarpet, Chennai - 600 018, Tamil Nadu, India. E-mail: balajikirushnan@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Immunoglobulin A (IgA) nephropathy is the most common glomerular disease worldwide. It usually presents as a nephritic syndrome with macroscopic hematuria, oliguria, and proteinuria with or without azotemia. Rapidly progressive glomerulonephritis with crescents is being described in about 30% of cases and is mostly associated with nephrotic-range proteinuria, accelerated hypertension, and accelerated decline toward end-stage renal disease. Medullary angiitis is a rare finding in renal biopsy and is usually associated with pauci-immune glomerulonephritis. We describe a rare association of medullary angiitis in IgA nephropathy, probably the first reported case in the country.
Keywords
IgA nephropathy
IgA vasculitis
medullary angiitis
The diagnosis of IgA nephropathy (IgAN) and reporting of relevant pathologic features in the renal biopsy report is made by synthesizing the IF and light microscopy. Renal medullary angiitis is defined as inflammation around the vasa recta of the medulla. It is not a common finding being reported in a cases of IgAN.
Case Report
A 50-year-old male with no comorbidities had a history of edema, purpuric rashes, and bilateral ankle pain since 4 days. He had consulted a dermatologist for the same. He was started on oral steroids tablet prednisolone 5 mg once a day for 1 week. Routine blood tests done elsewhere revealed serum creatinine of 3.5 mg%. He was referred to us. On examination, he had bilateral pitting pedal edema and purpuric rashes over the extensor surfaces of the hands and legs. His blood pressure was 160/100 mmHg, weight was 82 kg, and height was 168 cm. Investigations revealed urea of 254 mg%, creatinine of 11.08 mg%, albumin of 3.3 g%, albumin–globulin (AG) ratio of 0.9, uric acid of 9.83 mg%, potassium of 6.14 meq/L. Urine routine examination showed albumin 4+ with 20–25 RBCs (red blood cells)/hpf and granular casts. The urine spot protein to creatinine ratio was 16.8. He was taken up for hemodialysis through the right internal jugular catheterization. Two-dimensional (2D) echo showed normal left ventricular systolic function. He was given intravenous methylprednisolone 500 mg once a day for 3 days. Antinuclear antibody was negative by immunofluorescence. Anti-MPO (myeloperoxidase) IgG and Anti-PR3 (proteinase 3) IgG were negative by ELISA (enzyme-linked immunosorbent assay). Complement c3 was normal. Anti-GBM (glomerular basement membrane) antibody by ELISA was negative. Renal biopsy was done from the lower pole of the left kidney under local anesthesia.
Light microscopy showed five glomeruli with two being globally sclerosed. Viable glomeruli show mesangial hypercellularity. Segmental endocapillary hypercellularity with fibrocellular crescents was seen in two glomeruli. There was no spike formation or double contouring of the basement membrane. Interstitial fibrosis and tubular atrophy involved 15% to 20% of the cortex. There was an acute tubular injury. The interstitium was edematous and showed mild scattered inflammation in the cortex. No peritubular capillaritis or arteriolitis was identified in the cortical tissue. The medulla showed extensive interstitial hemorrhage with neutrophilic infiltrate and karyorrhectic debris [Figure 1 and Figure 2]. IF study showed IgA (3+), C3 (2+), kappa (+2) and lambda (+3) predominantly on the mesangium. IgG, IgM, and C1q (complement component 1q) were negative [Figure 3].
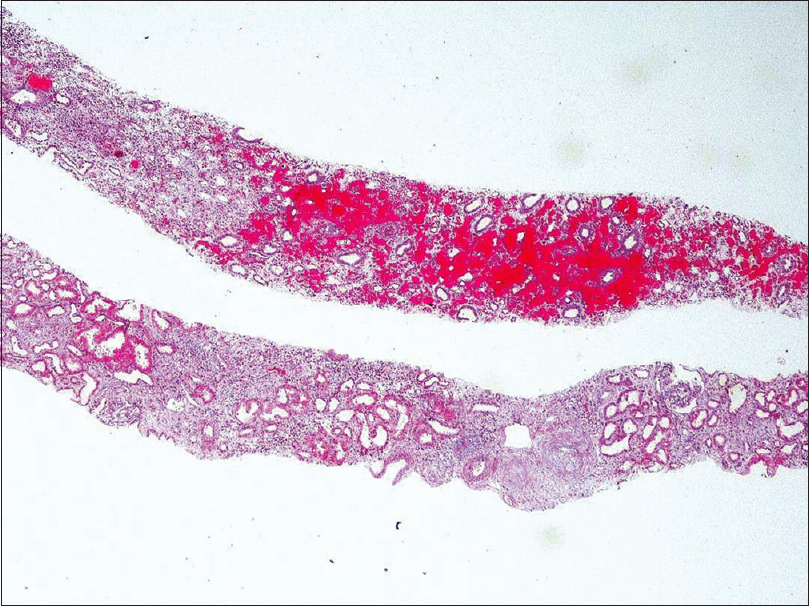
- Interstitial haemorrhage within the medulla . Massons trichrome 40X
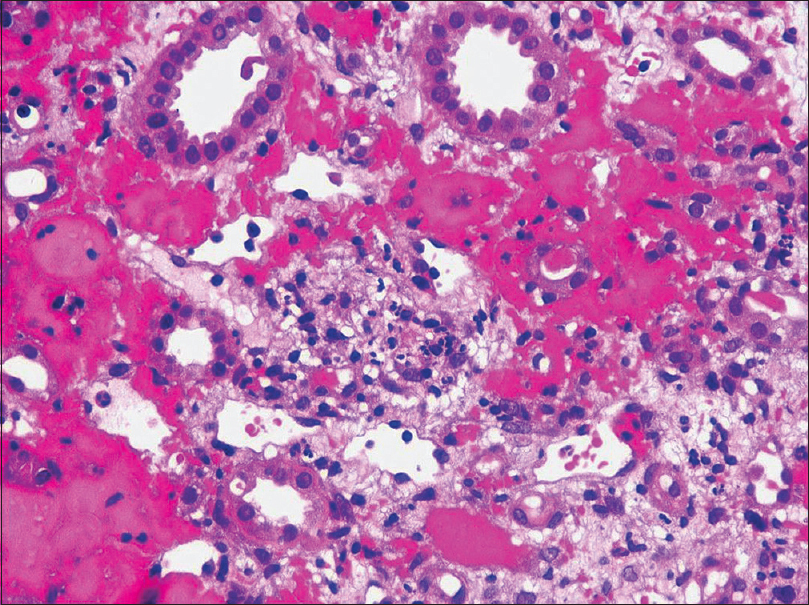
- Interstitial haemorrhage in the medulla with extravasated red blood cells, neutrophils and karyorrhectic debris. H and amp; E 400X
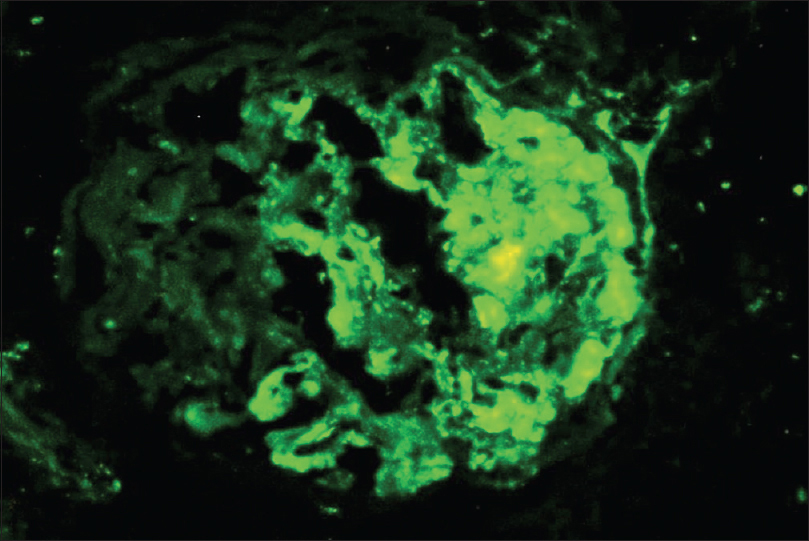
- Prominent deposits of IgA in the mesangium with segmental deposits along the glomerular basement membrane
He was continued on dialysis three times a week. Prednisolone 1 mg/kg was continued with the addition of tablet Mycept 500 mg twice daily. He is continued on hemodialysis with weekly monitoring of renal functions to assess the recovery of renal functions.
Discussion
IgA nephropathy (IgAN) most commonly affects males of Southeast Asian descent in the second or third decades of life, and it often presents with episodic macroscopic hematuria and mild proteinuria in the setting of tonsillitis or pharyngitis.[1] IgAN is usually a benign form of glomerulonephritis, with approximately 25% to 30% of patients reaching end-stage renal disease after 10 years.[1] Clinical risk factors linked to progressive IgA disease include hypertension, proteinuria >1.0 g/24 hours, male gender, and persistent microscopic hematuria.[2] Approximately 25%–30% of patients present with nephrotic syndrome and/or rapidly progressive glomerulonephritis.[3] Due to the frequently mild symptoms, the more indolent forms can remain undetected for years. Potential secondary causes should be excluded clinically. IgAV (IgA vasculitis) occurs primarily in children and presents with some combination of purpuric rashes, gastrointestinal pain, arthritis, and hematuria/proteinuria. The diagnosis of IgAN and reporting of relevant pathologic features in the renal biopsy report is made by synthesizing the IF and light microscopy. Renal medullary angiitis is defined as inflammation around the vasa recta of the medulla. Interstitial hemorrhage with associated polymorphonuclear leukocyte infiltration and karyorrhectic debris is found in the renal biopsy specimens. Necrotizing medullary lesions in the kidney biopsy have been reported in 1994 by Bonsib et al.[4] with associated vasculitis (ANCA). Fulminant cases of Wegener’s granulomatosis have revealed renal papillary necrosis by gross examination way back in 1983.[5] The largest of the case series was described by Hendricks et al.,[6] who reviewed 38 patients with medullary angiitis; among them 67% were identified as having AAV. In the same series, 20% of the patients had IgAN, and the remaining was associated with the use of antibiotics. The finding of medullary angiitis on renal biopsy should provoke further serologic testing in the case of crescentic glomerulonephritis. Drug-induced etiologies should be considered if serological markers like anti-MPO or anti-PR3 on ELISA are negative.[6] The most common non-ANCA etiology of medullary angiitis was IgAN in the above case series. It has not been reported in any other glomerular disease so far. The 2013 series was the largest medullary angiitis series described and the first to show medullary angiitis outside the setting of ANCA-associated disease. It is an important lesion to recognize as it frequently suggests the presence of systemic vasculitis and could be mistaken for interstitial nephritis. It correlates with severe disease and systemic vasculitis features. To the best of our knowledge, this is the first case report of medullary angiitis in IgAN from our country. Clinicians should be aware of this rare finding in IgAN and alert for possible severe systemic vasculitic involvement in renal limited disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am JKidney Dis. 2001;36:227-37.
- [Google Scholar]
- Early prediction of IgA nephropathy progression: Proteinuria and AOPP are strong prognostic markers. Kidney Int. 2004;66:1606-12.
- [Google Scholar]
- Necrotizing medullary lesions in patients with ANCA associated renal disease. Mod Pathol. 1994;7:181-5.
- [Google Scholar]
- Renal papillary necrosis associated with Wegener's granulomatosis. Hum Pathol. 1983;14:551-7.
- [Google Scholar]
- Renal medullary angiitis: A case series from a single institution. Hum Pathol. 2013;44:521-5.
- [Google Scholar]







