Translate this page into:
Invasive gastric mucormycosis and cytomegalovirus infection in an ABO incompatible renal transplant recipient
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Opportunistic infections are common in immunocompromised patients, such as solid organ transplant recipients. Both fungal and viral infections in posttransplant period increase morbidity and mortality. Cytomegalovirus (CMV) remains one of the most important pathogens. CMV disease may manifest as a nonspecific febrile syndrome or tissue-invasive infections. Zygomycosis is a rare infection, usually presents in rhino-cerebral, pulmonary and disseminated forms; gastrointestinal (GI) tract being a rare site of involvement. Newer techniques for early diagnosis and efficient therapies are essential for a better outcome of the disease; however, mortality rate remains high despite aggressive therapy. We report a renal transplant recipient, who developed gastric mucormycosis along with tissue invasive CMV disease, within 4 weeks of renal transplant and was diagnosed on the basis of upper GI endoscopy and gastric biopsy. The patient succumbed to the infection in spite of gastrectomy, antifungal and antiviral therapy.
Keywords
ABO incompatible
cytomegalovirus
mucormycosis
renal transplant
Introduction
Mucormycosis is a rare angioinvasive fungal infection, leading to vascular thrombosis, tissue infarction, necrosis and ulceration. The disease occurs almost exclusively in immunocompromised individuals though cases in immune-competent host have also been described.[12] Cytomegalovirus (CMV) is frequent in posttransplant period especially in those, who have received depleting antibodies. In addition to its directly attributable effects in the involved organs, active CMV disease has immunomodulatory effects, which predispose to other serious infections as well as acute rejections.
Gastrointestinal (GI) mucormycosis is rare and occurs primarily in the extremely malnourished patients especially infants or children and is thought to arise from ingestion of the fungi.[3] Presence of CMV infection in GI tract may trigger invasive fungal infections in renal transplant recipient by its immunomodulatory effects. Early diagnosis requires a high index of suspicion and an emergent evaluation to allow early initiation of antifungal/antiviral and surgical therapy.
Case Report
A 45-year-old male with end-stage renal disease (ESRD) underwent a live donor ABO-incompatible renal transplant (recipient - B positive, donor - A positive) with wife as donor. He was negative for hepatitis B, C and human immunodeficiency virus infections pretransplant. Both recipient and donor were seropositive for CMV. For preconditioning, he received rituximab (200 mg) followed by two sessions of double filtration plasmapheresis and IV immunoglobulin (10 g) to decrease the Anti A titers from 1:64 to <1:8 (target titers for our center). Tacrolimus (0.05 mg/kg/day) and mycophenolate (1000 mg/day) was started 1-week prior to transplant. Pretransplant complement-dependent cytotoxic crossmatch and flow cytometric crossmatch were negative. Patient received two doses of basiliximab. In postoperative period, there was initial diuresis; however, 6 h after surgery, urine output dropped. Color Doppler flow imaging showed no evidence of arterial or venous thrombosis with raised resistive indices. In view of persistently low urine output despite adequate fluid resuscitation, re-exploration was done. The graft was turgid with normal anastomosis. Wedge graft biopsy was taken. On postoperative day 1, patient developed drop in hemoglobin (10.7 g/dl to 7.7 g/dl) and platelets (from 1.2 lacs/cmm to 50,000/cmm) with evidence of schistocytes on peripheral smear and biochemical evidence of hemolysis. Biopsy showed diffuse acute thrombotic microangiopathy (TMA). Tacrolimus trough level was 22.3 ng/ml. In the absence of positive C4d staining and other histological evidence suggestive of antibody-mediated rejection, the TMA was thought to be tacrolimus induced. Hence, tacrolimus was stopped, and plasma exchanges were started. Patient received five sessions of plasmapheresis over next 10 days. Anti A titer remained 1:4 postoperatively. Patient developed delayed graft function requiring dialytic support.
On 1-week posttransplant, he developed generalized tonic-clonic seizures with respiratory distress requiring intubation and mechanical ventilation. Brain imaging and electroencephalogram were normal. Seizures were thought to be secondary to TMA, hence plasma exchanges were continued. Subsequently, during ICU stay, patient developed septic shock requiring broad spectrum antibiotics. Mycophenolate was stopped in view of life-threatening sepsis. His condition gradually improved with an increase in urine output and decreasing serum creatinine levels. Patient was gradually weaned off the ventilator and dialysis was also stopped (serum creatinine 3.1 mg/dl). He was switched to cyclosporin and steroids were continued.
On 4-week posttransplant, patient developed non colicky epigastric pain along with recurrent non bilious vomiting. Physical examination revealed tenderness in epigastrium and right hypochondrium. Examination of renal graft and other system was unremarkable. Lab investigations revealed pancytopenia. Liver function test, serum amylase, lipase and blood sugar level were normal. Ultrasonography of the abdomen was normal. C0 level was 68.90 ng/ml.
In view of recurrent vomiting, upper GI endoscopy was done, which revealed deep, round punched out hemorrhagic ulcerations involving the esophagus and corpus of the stomach along with necrotic-ulcerative lesions involving the lesser curvature of the stomach. Gastric biopsy from the involved regions revealed broad, nonseptate hyphae with right angled branching on sections stained with H and E and grocotts stain, suggestive of mucormycosis [Figure 1]. Also, there was evidence of intranuclear inclusion bodies in gastric mucosa suggestive of CMV gastritis, which was confirmed by immunohistochemistry [Figure 1]. Blood CMV viral load was 2200 copies/ml.
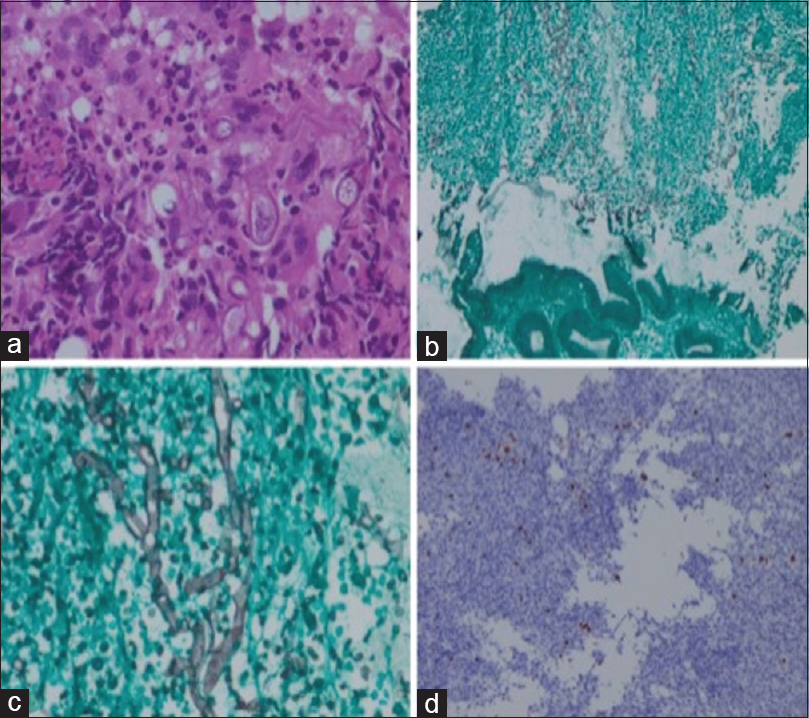
- (a) Higher magnification reveals broad nonseptate mucor hyphae (original magnification, H and E, ×40). (b) grocotts stain reveals gastric mucosal epithelial fragment at the lower end and necrotic suppurative exudate at the upper end (original magnification, Grocotts, ×4). (c) Higher magnification of the necrotic area reveals broad nonseptate hyphae of mucormycosis (original magnification, Grocotts, ×40). (d) Cytomegalovirus (CMV) immunostain shows nuclear positivity in the affected cytomegalic cells (original magnification, CMV IHC, ×20)
Patient was started on intravenous liposomal amphotericin B (3 mg/kg/day) and ganciclovir (2.5 mg/kg/day). Cyclosporin was stopped. After 2 days, he developed severe abdominal pain with hypotension. Computed tomography scan of the abdomen showed pneumoperitoneum and dehiscence of posterior wall of the stomach with leak of contents. Patient was taken for urgent exploratory laparotomy, which revealed 3 cm × 3 cm rent in the posterior wall of the stomach. Distal gastrectomy along with debridement and feeding jejunostomy was done. Postoperatively he remained on inotropic, ventilatory and dialytic support. Liposomal amphotericin B was continued along with ganciclovir and antibiotics. However, his condition progressively worsened, and he succumbed to the infection. The course of illness is shown in Figure 2.
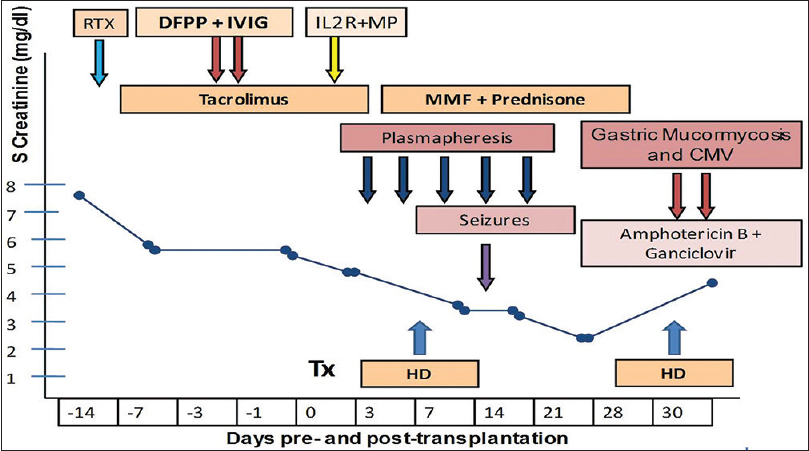
- Timeline of events during course of illness. (RTX - Rituximab, DFPP - Double filtration plasmapheresis, IL2RA - Interleukin 2 receptor antagonist, MP - Methyl prednisone, HD - Hemodialysis)
Discussion
Renal transplantation is the best treatment modality for patients with ESRD. Traditionally, renal transplant across blood groups was considered as contraindication in the past; however, with the availability of present day immunosuppression, this no longer holds true. The commonly used triple-drug immunosuppression along with induction therapy has improved graft survival but leaves the patient susceptible to a variety of opportunistic infections, of which fungal and viral infections are the leading cause of morbidity and mortality.[4]
Cytomegalovirus infection remains the most common viral infection postrenal transplant patients.[5] The reported incidence of opportunistic fungal infections is 6–10% of all recipients of solid organ transplants in India.[67] Mucormycosis is a rare life-threatening infection caused by fungi; classified as zygomycetes. Invasive GI mucormycosis is extremely rare and occurs almost always in malnourished or immunocompromised patients. Stomach is the most common site of involvement, followed by colon. Tissue invasion is characterized by necrosis, ulceration, perforation, and hemorrhagic shock. Mortality rate of disease remains high despite aggressive therapy.[3]
The first case of GI mucormycosis in a renal transplant recipient was described by Winkler et al., in a patient who had received antirejection therapy with steroids and anti thymocyte globulin in postoperative period, making her a susceptible candidate for infections. This patient was successfully managed with amphotericin B and omeprazole.[8] There have been very few case reports of gastric mucormycosis in renal transplant recipients in last decade.[91011]
Our patient developed invasive gastric mucormycosis within 4 weeks of transplant with severe dyspeptic symptoms and was diagnosed on the basis of upper GI endoscopy and biopsy. Patient also had evidence of coexistent CMV infection on gastric biopsy, which probably predisposed to the fungal infection in this patient. Both fungal and CMV infections are unusual in the 1st month postrenal transplant. This patient developed mixed mucormycosis and CMV infections, probably due to over immunosuppression. Being ABO incompatible transplant, he received rituximab and plasmapheresis as preconditioning therapy and received interleukin 2 receptor antagonist as induction therapy. His initial tacrolimus levels were in the higher range. He also received prolonged high doses of steroids, as he was off tacrolimus and mycophenolate in view of tacrolimus-induced TMA and sepsis. The occurrence of CMV infection early in the course can be attributed to over immunosuppression, which may predispose to other opportunistic infections by its indirect effects like invasive fungal infection in this case. The portal of inoculation for mucormycosis in our patient was GI tract. This can be due to multiple intubations including Ryle's tube, which may have acted as portal of entry. The course of the disease was rapidly fatal due to angio-invasive nature of mucormycosis in spite of surgical exploration and debridement along with antifungal and antiviral treatments at appropriate time.
There are very few reports of mixed CMV and mucormycosis in renal transplant recipients. Ju et al., reported a case of renal transplant recipient, who received antirejection therapy with steroids and OKT3 on postoperative day 18 and presented with sudden onset massive hematochezia. Colonoscopy revealed multiple colonic ulcers and histopathology was consistent with mucormycosis and CMV infection. This patient responded to 5 weeks therapy of amphotericin B and ganciclovir therapy.[12] However, the disease was fatal in our patient in spite of gastrectomy and treatment with liposomal amphotericin B and ganciclovir. Andrews et al., reported two cases of renal transplant recipient with CMV infection, who had received anti thymocyte globulin for induction therapy and high doses of steroids for rejection.[13]
In renal transplant recipients with mucormycosis, immunosuppression needs to be modified. Successful outcome of disease depends upon early tissue diagnosis, efficient antifungal therapy and surgical debridement of necrotic tissue if indicated. Medical therapy of mucormycosis with amphotericin B remains the standard of care. Posaconazole is the only new antifungal drug with potential activity against these molds.[1415] The treatment of established CMV disease requires valganciclovir or intravenous ganciclovir until CMV replication is no longer detected.
Conclusion
The morbidity and mortality of mucormycosis and CMV infection in renal transplant recipient remains high. There are very few cases of mixed CMV and mucormycosis in renal transplant recipients in the literature. Improvement in patient outcome requires preventive strategies and early management of infection on emergent basis.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556-69.
- [Google Scholar]
- Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997;10:86-124.
- [Google Scholar]
- Human cytomegalovirus and kidney transplantation: A clinician's update. Am J Kidney Dis. 2011;58:118-26.
- [Google Scholar]
- High mortality in systemic fungal infections following renal transplantation in third-world countries. Nephrol Dial Transplant. 1993;8:168-72.
- [Google Scholar]
- Gastric mucormycosis due to Rhizopus oryzae in a renal transplant recipient. J Clin Microbiol. 1996;34:2585-7.
- [Google Scholar]
- Time table of infections after renal transplantation – South Indian experience. Indian J Nephrol. 2005;15:S14-21.
- [Google Scholar]
- Nonfatal gastric mucormycosis in a renal transplant recipient. South Med J. 1997;90:341-4.
- [Google Scholar]
- Successful treatment of massive lower gastrointestinal bleeding caused by mixed infection of cytomegalovirus and mucormycosis in a renal transplant recipient. Am J Nephrol. 2001;21:232-6.
- [Google Scholar]
- Mucormycosis in transplant recipients: Possible case-case transmission and potentiation by cytomegalovirus. Nephrol Dial Transplant. 1994;9:1194-6.
- [Google Scholar]
- Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob Agents Chemother. 2003;47:3647-50.
- [Google Scholar]
- Successful nonoperative management of gastrointestinal mucormycosis: Novel therapy for invasive disease. Surg Infect (Larchmt). 2009;10:447-51.
- [Google Scholar]







