Translate this page into:
Light chain proximal tubulopathy with cast nephropathy in a case of multiple myeloma
Address for correspondence: Dr. Kiran Krishne Gowda, Department of Histopathology, PGIMER, Chandigarh, India. E-mail: kicha.doc.2k@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The renal diseases most frequently associated with myeloma include cast nephropathy (CN), amyloidosis and monoclonal immunoglobulin deposition disease. Light chain proximal tubulopathy (LCPT) is reported less frequently. Majority of the cases with κ-restriction present with Fanconi syndrome (FS) and show crystals in proximal tubular epithelial cytoplasm. In contrast, those with λ-restriction are infrequently associated with FS and show cytoplasmic vacuolations in proximal tubular epithelial cytoplasm. Combination of morphologies in kidney affected by plasma cell dyscrasias is rare and co-existence of LCPT and CN is one of the rarest. We report a case of multiple myeloma having this rare combination of morphologies.
Keywords
Cast nephropathy
fanconi syndrome
proximal tubulopathy
Introduction
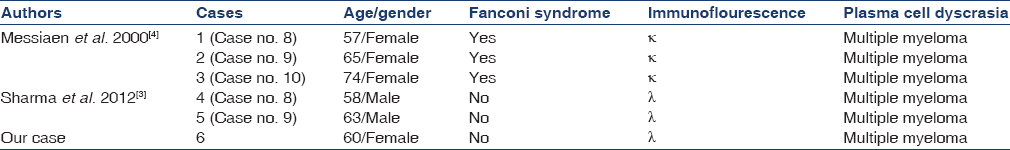
Common morphological patterns of kidney involvement in multiple myeloma include cast nephropathy (CN), amyloidosis and monoclonal immunoglobulin deposition disease (MIDD). Less frequent forms include: Immunotactoid glomerulonephritis, neoplastic plasma cell infiltration, interstitial nephritis, and light chain proximal tubulopathy (LCPT). In LCPT, excessive LCs are excreted through the kidney and are reabsorbed in the proximal tubular cells leading to tubular damage, frequently resulting in acquired Fanconi syndrome (FS). Although LCPT with λ LC is less frequently documented in literature, recent publications emphasize the difficulties in its detection rather than lack of its existence.[123] Here, we describe a case of multiple myeloma having combination of LCPT with CN without FS. Combination of different patterns of renal involvement is rare, with most common being MIDD with CN.[2] Only five cases of LCPT with CN have been documented, this being sixth such case.[34]
Case Report
A 60-year-old female presented with complaints of loss of appetite, easy fatigability and body ache since 2 months. She had no history of fever, dysuria, flank pain, hematuria, oral ulcers or swelling of lower limbs/facial puffiness. On examination, her pulse rate was 80/min and blood pressure was 100/70 mm of Hg with no icterus, cyanosis, clubbing or pedal edema. Musculoskeletal examination revealed bony tenderness. Systemic examination was unremarkable. Laboratory investigations revealed Hb of 60 g/l, total leukocyte count of 4.7 × 109/l and platelet count of 147 × 109/l. Renal function tests revealed renal dysfunction with blood urea of 40 mg/dl and serum creatinine of 1.9 mg/dl. Serum calcium and phosphorus were 12.84 mg/dl and 3.4 mg/dl, respectively. Serum intact parathyroid hormone was 20 pg/ml. Liver function tests revealed aspartate aminotransferase/alanine transaminase, alkaline phosphatase, total protein/albumin and total bilirubin of 24/35 IU/ml, 83 IU/ml, 6.3/4.1 g/dl and 1.08 mg/dl, respectively. Her antinuclear antibody and antineutrophil cytoplasmic antibody serology were negative. Serum complement levels were normal. Serum for hepatitis B surface antigen and antibody for hepatitis C virus and HIV-I/II were negative. Urine examination showed albuminuria and 1–3 white blood cells/high-power field with no glycosuria. Her 24 h urine protein was 1.2 g. With a provisional diagnosis of rapidly progressive renal failure, kidney biopsy was performed.
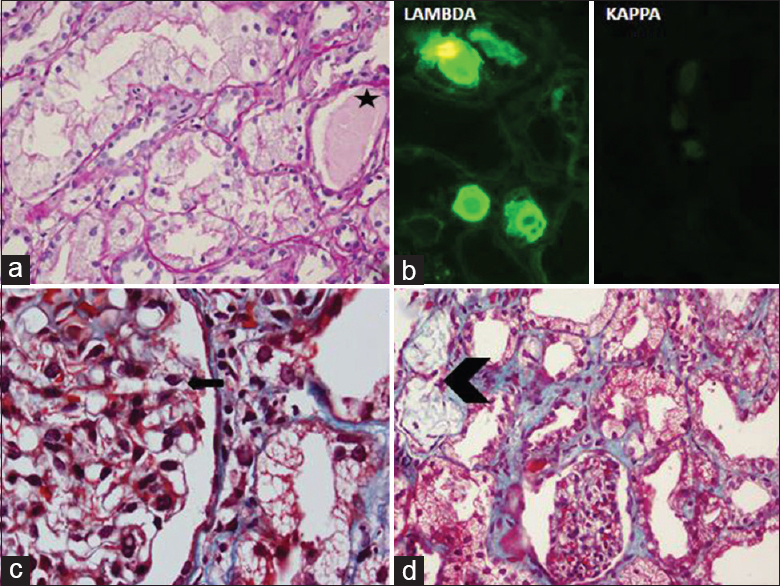
Kidney biopsy showed changes predominantly in tubulointerstitial compartment. Proximal tubules showed marked cytoplasmic vacuolations, which were periodic acid-Schiff (PAS)-negative, but fuchsinophilic [Figure 1a and b]. No crystals or crystalloids were noted. On direct immunofluorescence cytoplasmic vacuoles revealed staining for λ LC, while κ LC was negative [Figure 1c and d]. Distal tubules showed casts demonstrating λ restriction [Figure 2a and b]. The glomeruli (n = 12) showed occasional podocytes with cytoplasmic vacuolations [Figure 2c] and negative staining results for immunoglobulins, complement, and both LCs on immunofluorescence (n = 5). Focal interstitial collections of foamy histiocytes were noted [Figure 2d]. Thus, LCPT with CN having λ LC restriction was diagnosed.

- Light microscopy shows vacuolations in the proximal tubular epithelial cytoplasm. (a) H and E stain, ×400, which reveal fuchsinophilic droplets. (b) Masson's trichrome stain, ×1000. Direct immunofluorescence microscopic examination shows staining for λ light chain within the proximal tubular cytoplasm, while κ light chain are undetectable. (c) λ light chain, (d) κ light chain; ×200
- Distal tubules showing fractured periodic acid-Schiff (PAS)-negative casts (star), which on direct immunofluorescence microscopic examination demonstrated λ light chain restriction. (a) PAS stain, ×400. (b) DIF of λ and κ light chains, ×200. Podocytes revealing cytoplasmic vacuolations (arrow) and focal collections of foamy histiocytes (arrow head) can also be noted. (c and d) Masson's trichrome stain, ×1000 and × 200, respectively
In view of the diagnosis, patient was investigated for clonal hematopoietic disorder. Skeletal survey showed multiple lytic lesions in vertebrae. Serum protein electrophoresis did not show monoclonal proteins. Urine electrophoresis showed monoclonal protein in β-γ region. Free LC assay showed serum κ and λ of 2.61 mg/dl and 2940 mg/dl, respectively (ratio of κ/λ - <0.01). Bone marrow examination revealed 43% plasma cells with expression of CD138 and λ thus confirming multiple myeloma. There was no glucosuria, aminoaciduria, bicarbonaturia, phosphaturia, or hyperuricosuria to suggest FS.
With a diagnosis of multiple myeloma manifesting with LCPT and CN, treatment was started with combination chemotherapy of bortezomib, thalidomide and dexamethasone along with plasmapheresis. At completion of therapy at the end of 6 months, patient had normalization of κ/λ ratio with serum creatinine of 0.9 mg/dl and normal urine examination.
Discussion
First description of LCPT causing FS with documentation of needle-shaped crystals in proximal tubular epithelial cell cytoplasm by electron microscopy was in 1957.[5] Subsequently <100 cases of LCPT have been documented in English literature.[12346] The largest series of 17 cases was reported by Maldonado et al. in 1975.[7] LCPT is not restricted to cases with plasma cell dyscrasias and has been demonstrated in non-Hodgkin lymphomas like diffuse large B-cell lymphoma,[6] Burkitt lymphoma[8] and Waldenstrφm macroglobulinemia.[9] Patients may have varied clinical presentations such as FS, kidney failure, proteinuria or osteomalacia. A vast majority of reported cases are associated with full-blown or incomplete FS. Very few cases of LCPT without FS (nine — including present case) have been reported.[31011] It is interesting to note that all cases of LCPT without FS show λ restriction.
Among cases of LCPT, only 26 (including present case) with λ restriction have been documented.[1238] Unlike LCPT cases with κ restriction, majority of which show intracytoplasmic crystals in proximal tubules, those with λ restriction have varied morphology. Morphology on light microscopy varies from presence of crystals,[12] cytoplasmic vacuolations to just changes of acute tubular injury. This morphologic heterogeneity has been well-documented by Larsen et al.[2] who believe that cases of λ-restricted LCPT are much more common than previously reported, possibly due to absence of crystals and difficulty in identifying LC restriction in tubules on immunofluorescence. They demonstrated LC restriction is better by immunohistochemistry on paraffin-embedded sections than by immunofluorescence of frozen sections. They reasoned this was due to better accessibility of antigens after the antigen extraction steps in immunohistochemistry. Although electron microscopy was not done in the index case, crystals were not noted on light microscopy, direct immunofluorescence or semi-thin sections.
LCPT can occasionally be accompanied by CN[34] with six such cases [Table 1] being documented till date (including present case). Only three of these cases presented with FS and showed κ restriction. Development of proximal tubular dysfunction in cases of paraproteinemia depends on multiple factors. Normally free LCs are filtered by glomerulus and reabsorbed by proximal tubule, where they are degraded by cathepsin B and pepsin, lysosomal enzymes. Abnormal LCs secreted by neoplasms differ in Vκ domain, making them resistant to proteolysis by lysosomal enzymes.[13] LCs from patients with LCPT characteristically exhibit this property. In addition, excess free LCs secreted by neoplastic cells overload proximal tubular lysosomal system. These tubulopathic LCs have been shown to have low affinity for Tamm-Horsfall proteins, unlike LCs of CN.[1415] The abnormal κ LCs in cases of LCPT have tendency to spontaneously crystallize, unlike λ LCs, which lead to lysosomal dilatation without crystal formation. However, borderline cases in which LCs share both properties could explain cases of CN with LCPT. The histological changes in renal biopsy in the present case are subtle and may not be appreciated or may be missed, especially when LC deposits are not detected by routine immunoflourescence. It is important to distinguish between protein reabsorption droplets and vacuolations, with the former being PAS-positive. The index case showed PAS-negative cytoplasmic vacuolations of proximal tubular epithelium throughout biopsy, with few myeloma casts in distal tubules. Immunoflourescence showed tiny droplets corresponding to vacuoles in proximal tubules and positive casts with λ. Renal failure in this patient was predominantly due to LCPT than CN, because of the focal nature of the latter.
In summary, we document a rare case of LCPT with CN showing λ restriction in patient of multiple myeloma. Establishment of pathogenesis and clinical relevance of such rare combination needs further studies.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Expanding the pathologic spectrum of immunoglobulin light chain proximal tubulopathy. Arch Pathol Lab Med. 2007;131:1368-72.
- [Google Scholar]
- The morphologic spectrum and clinical significance of light chain proximal tubulopathy with and without crystal formation. Mod Pathol. 2011;24:1462-9.
- [Google Scholar]
- Light chain proximal tubulopathy: Expanding the pathologic spectrum with and without deposition of crystalline inclusions. ISRN Pathol. 2012;2012:1-6.
- [Google Scholar]
- Adult Fanconi syndrome secondary to light chain gammopathy. Clinicopathologic heterogeneity and unusual features in 11 patients. Medicine (Baltimore). 2000;79:135-54.
- [Google Scholar]
- Multiple myeloma and the adult Fanconi syndrome. I. Report of a case with crystal-like deposits in the tumor cells and in the epithelial cells of the kidney. Am J Med. 1957;22:5-23.
- [Google Scholar]
- Fanconi syndrome in adults. A manifestation of a latent form of myeloma. Am J Med. 1975;58:354-64.
- [Google Scholar]
- Light chain proximal tubulopathy without crystals in a case of Burkitt lymphoma presenting with acute kidney injury. Am J Kidney Dis. 2013;62:638-41.
- [Google Scholar]
- Fanconi's syndrome induced by a monoclonal Vkappa3 light chain in Waldenstrom's macroglobulinemia. Am J Kidney Dis. 2005;45:749-57.
- [Google Scholar]
- Light chain tubulopathy without Fanconi syndrome. Nephrol Dial Transplant. 2006;21:3589-90.
- [Google Scholar]
- Plasma cell dyscrasia causing light chain tubulopathy without Fanconi syndrome. Am J Kidney Dis. 2010;55:1136-41.
- [Google Scholar]
- Lambda light chain induced nephropathy: A rare cause of the Fanconi syndrome and severe osteomalacia. Am J Kidney Dis. 1998;32:E3.
- [Google Scholar]
- Protease resistance and binding of Ig light chains in myeloma-associated tubulopathies. Kidney Int. 1995;48:72-9.
- [Google Scholar]
- Localization of a single binding site for immunoglobulin light chains on human Tamm-Horsfall glycoprotein. J Clin Invest. 1997;99:732-6.
- [Google Scholar]
- Mechanisms of intranephronal proteinaceous cast formation by low molecular weight proteins. J Clin Invest. 1990;85:570-6.
- [Google Scholar]







