Translate this page into:
Nephrotic Syndrome and Posterior Reversible Encephalopathy Syndrome as Clinical Presentations of Gemcitabine-Induced Thrombotic Micro-Angiopathy
Corresponding Author: Dr. Vijoy Kumar Jha, Physician and Nephrologist, Command Hospital Air Force, Bangalore - 560 007, Karnataka, India. E-mail: vkjhamd@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jha VK, Akal RS, Mahapatra D, Sharma A, Singh BP, Arora R. Nephrotic Syndrome and Posterior Reversible Encephalopathy Syndrome as Clinical Presentations of Gemcitabine-Induced Thrombotic Micro-Angiopathy. Indian J Nephrol 2024;34:74-8. doi: 10.4103/ijn.ijn_277_22
Abstract
Gemcitabine-induced thrombotic micro-angiopathy (GiTMA) is a very rare pathology of micro-vascular occlusion with a poor prognosis. In this case report, we present a young male with pancreatic carcinoma who received gemcitabine as adjuvant chemotherapy and developed thrombotic micro-angiopathy (TMA) manifesting as nephrotic syndrome with renal dysfunction and posterior reversible encephalopathy syndrome (PRES). The case was successfully managed with discontinuation of the drug and conservative management. The pathogenesis of GiTMA might be direct endothelial dysfunction with consequent activation of the clotting system. The role of plasma exchanges and monoclonal antibodies is unclear in drug-induced TMA.
Keywords
Gemcitabine
nephrotic syndrome
PRES
TMA
Introduction
Gemcitabine-induced thrombotic micro-angiopathy (GiTMA) is an uncommon adverse event with the pathology of micro-vascular thrombosis, leading to end organ damage. Drug-induced TMA manifesting as nephrotic syndrome with progressive renal dysfunction and posterior reversible encephalopathy syndrome (PRES) is not known. In this case report, we present a young male diagnosed with periampullary carcinoma who developed features suggestive of systemic TMA, presenting with worsening renal functions, nephrotic syndrome, and PRES. The relationship between drug-induced TMA and PRES is not clear.
Case Report
A 40-year-old male was diagnosed with periampullary carcinoma while being evaluated for generalized pruritus and high-colored urine of 2 weeks duration. He had features of obstructive jaundice with conjugated hyperbilirubinemia (total –13 mg/dl, direct bilirubin –9.2 mg/dl) and serum alkaline phosphatase (SAP) of 344 IU/L. Ultrasonography (USG) abdomen revealed bilobar intra-hepatic biliary radicle dilatation (IHBRD) with a dilated common bile duct (CBD), with the level of obstruction at the level of terminal CBD. His initial contrast-enhanced computed tomography (CECT) abdomen and magnetic resonance cholangio-pancreaticogram (MRCP) revealed features of IHBRD and CBD dilatation with an abrupt cut-off at the ampullary region, suggestive of distal CBD stricture. There was beading of intra-hepatic ducts, narrowing/stricture at branch points, and duct wall enhancement. Upper gastro-intestinal (UGI) endoscopy was suggestive of ulcero-proliferative growth in the ampulla. An endoscopic retrograde cholangio-pancreaticogram (ERCP) revealed a bulky and ulcerated ampulla. Biopsy of ulcerated growth was suggestive of adenocarcinoma. The patient underwent Whipple’s pancreaticoduodenectomy, and histopathology of the specimen confirmed moderately differentiated periampullary adenocarcinoma. He was given adjuvant chemotherapy with gemcitabine 3 cycles (total –3500 mg/m2).
One week after completion of the third cycle of gemcitabine, he developed generalized swelling of the body and progressive breathlessness. The evaluation revealed raised blood pressure (BP), pedal edema, and bilateral pleural effusion. He had laboratory features suggestive of nephrotic syndrome (serum albumin –1.5 gm/dl, total cholesterol –380 mg/dl, urine routine examination/microscopic examination – protein 3+, 5-6 RBC/high power field, and 24 hr urinary protein –8.8 gm). The other features on USG kidney ureter bladder (KUB), right kidney, 10.8 cm, and left kidney, 11 cm, with reduced corticomedullary differentiation (CMD). He had a progressive rise in serum creatinine from a baseline of 0.9 mg/dl to 3.4 mg/dl over 2 weeks. He also had bicytopenia (Hb of 7.2 gm/dl with a platelet count of 80,000/mm3). A peripheral blood smear was suggestive of micro-angiopathic hemolytic anemia (MAHA) and schistocytes. Lactate dehydrogenase (LDH) was raised (1274–878 IU/L). Other investigations revealed negative direct and indirect coombs test, normal iron profile, Vit B12, a normal folic acid level, negative auto-immune markers (ANA, anti-dsDNA, and ANCA), and negative viral markers (HIV, HBsAg, and anti-HCV). The coagulation profile was normal. The ADAMTS 13 activity was 86.30%, and anti-PLA2R Ab was negative (<2.0 RU/ml). Chest X-ray postero-anterior view revealed bilateral pleural effusion. The electrocardiogram (ECG) and 2D echocardiogram (Echo) were normal. The given features were suggestive of systemic TMA and nephrotic range proteinuria with progressive renal dysfunction, and he underwent a kidney biopsy. Light microscopy of the renal biopsy specimen revealed glomeruli with ischemic changes of varying severity, including fluffy appearing mesangial areas containing fragmented RBCs, wrinkled capillaries, loss of mesangial anchorage resulting in ectatically dilated and congested capillary lumina, mesangiolysis, and retraction of capillary tufts. Several glomeruli showed capillary wall double contour formation. Focal extra capillary proliferation (prominence of visceral epithelial cells) was also noted. Interstitial fibrosis and tubular atrophy (IFTA) were about 15% of the sampled cortex. Arteries showed mild medial thickening. Occasional arterioles showed necrotizing lesions with the presence of fragmented RBC and luminal thrombotic occlusion, suggestive of TMA [Figure 1]. Direct immunofluorescence (DIF) was negative. Electron microscopy [Figure 2] revealed loss of glomerular endothelial fenestrations and marked expansion of the subendothelial area and paramesangial electron-dense deposits, indicative of severe endothelial injury.
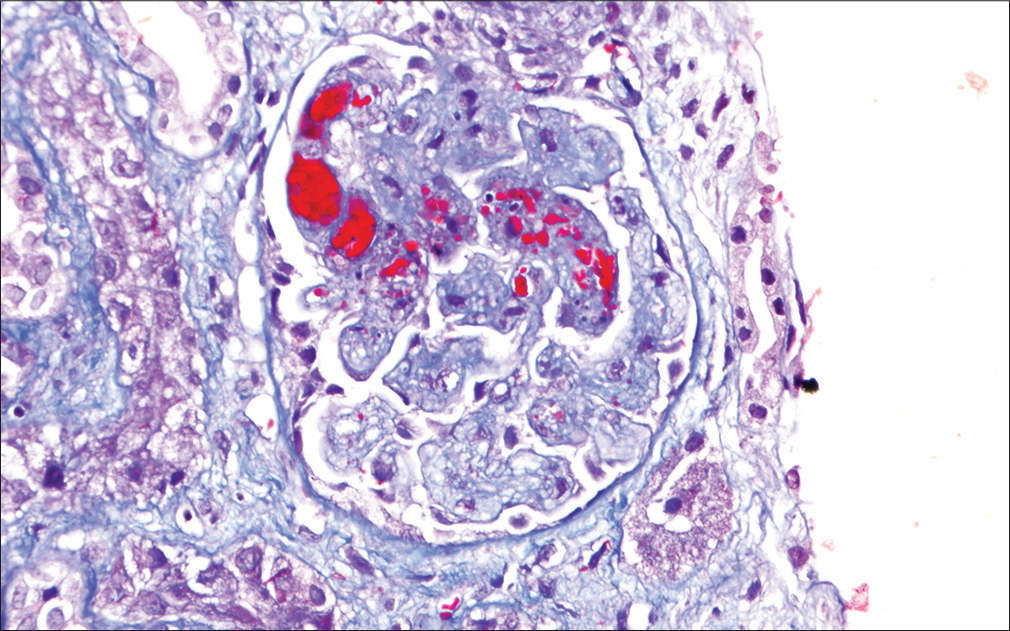
- Light microscopy photo-micrograph showing glomerulus with mesangiolysis, intra-capillary fibrin thrombi, and the presence of fragmented RBCs in glomerular capillary lumina and mesangial areas (thrombotic micro-angiopathy) (Masson’s TrichromeX200).
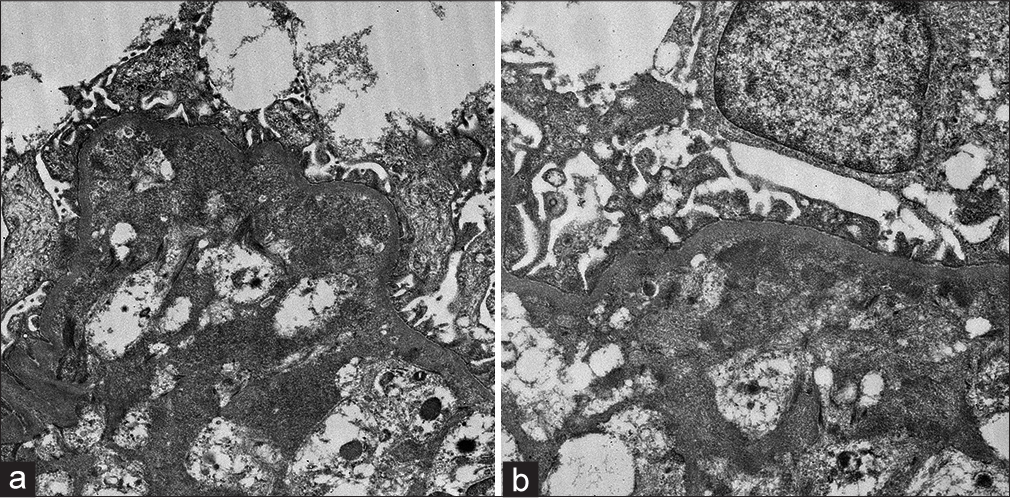
- (a) Electron microscopy image showing loss of glomerular endothelial fenestrations and a marked expansion of the subendothelial area indicative of severe endothelial injury (EM X 2500) (b) Electron microscopy image showing paramesangial electron-dense deposits (EM X4000).
He was managed by stopping the chemotherapy, and supportive treatment was given considering the diagnosis as a drug (gemcitabine)-induced TMA. His blood pressure was adequately controlled with two anti-hypertensives. One week post renal biopsy (3 weeks after completion of the third cycle of chemotherapy), he developed two episodes of generalized tonic-clonic seizure and had a prolonged acute confusional state in the postictal period. Magnetic resonance imaging (MRI) [Figure 3a and 3b] revealed multiple T2/FLAIR hyper-intensities of variable sizes in bilateral cerebral hemispheres, predominantly in the bilateral ganglio-capsular region (left > right) and bilateral occipital lobes with minimal mass effect evident in the form of chinking of bilateral lateral ventricles adjacent to the lesions. These findings were suggestive of atypical PRES.
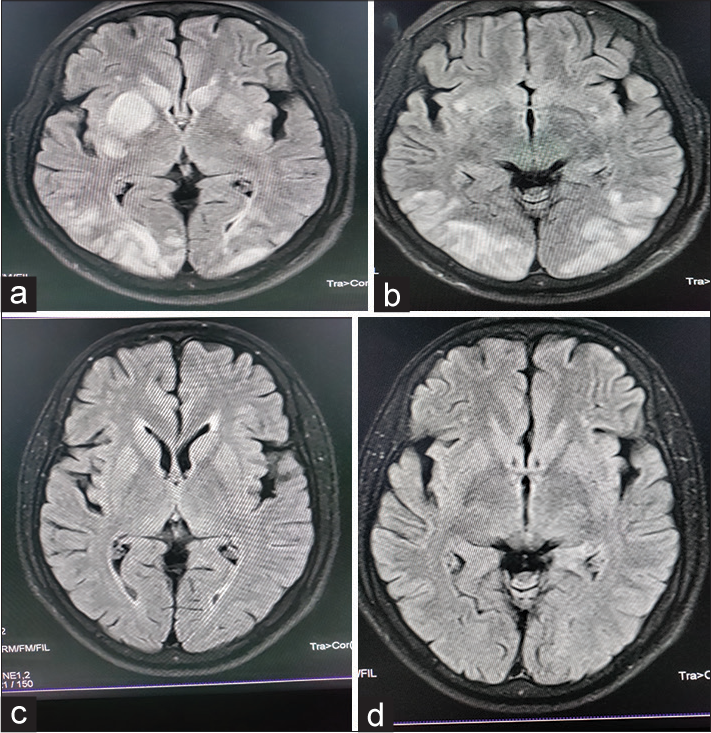
- (a and b) MRI revealed multiple T2/FLAIR hyper-intensities of variable sizes in bilateral cerebral hemispheres, predominantly in the bilateral ganglio-capsular region (left > right) and bilateral occipital lobes, with minimal mass effect evident in the form of chinking of bilateral lateral ventricles adjacent to the lesions. (c and d) MRI revealed complete resolution of T2/FLAIR hyper-intensities in bilateral occipital regions and the left ganglio-capsular region with complete resolution of the mass effect. There was minimal residual hyper-intensity in the right ganglio-capsular region.
He was put on anti-epileptic drugs, titration of anti-hypertensive drugs, and other supportive measures. Four weeks after this episode, a repeat MRI [Figure 3c and 3d] revealed complete resolution of T2/FLAIR hyper-intensities in bilateral occipital regions and left ganglio-capsular regions with complete resolution of the mass effect. There was minimal residual hyper-intensity in the right ganglio-capsular region. His renal dysfunction became stable after 3–4 weeks with serum creatinine ranging from 2.5 to 3.0 mg/dl with adequate urine output. Thereafter, he was also put on anti-proteinuric therapy (angiotensin receptor blocker telmisartan), calcium channel blocker (diltiazem), and other supportive measures. A repeat whole body positron emission tomography scan revealed no evidence of the disease. Follow-up after 3 months of anti-proteinuric therapy revealed Hb of 11 gm/dl, platelet of 200,000/mm3, LDH of 300 IU/L, no schistocytes on PBS, moderate proteinuria (urine RE/ME – Protein 2+, 2–3 RBCs/HPF, 24 hr urinary protein –1.8 gm), serum creatinine of 2.1 mg/dl, and serum albumin of 3.6 gm/dl. His blood pressure was adequately controlled with three anti-hypertensive drugs including an angiotensin receptor blocker and a calcium channel blocker.
Discussion
TMA is a potentially life-threatening condition caused by micro-vascular thrombosis in small vessels and associated with micro-angiopathic hemolytic anemia (MAHA), consumptive thrombocytopenia, and organ injury with platelet-induced micro-vascular occlusion (acute kidney injury, neurologic abnormalities, and/or cardiac ischemia). Nearly all TMAs cause MAHA and thrombocytopenia. The initial evaluation of TMA should focus on confirming MAHA and thrombocytopenia and excluding systemic disorders such as severe hypertension, disseminated intra-vascular coagulation, malignancy, infections, rheumatic disease, pregnancy, post-hematopoietic cell/solid organ transplantation, and so on.
Drug-induced TMA (DITMA), either immune or toxic, is one of the primary TMAs. It is very challenging to diagnose as the role of the potentially implicated drug may not be clear, and specific laboratory tests to identify a drug etiology may not be possible. In immune-mediated DITMA, multiple cellular targets of the drug-dependent antibodies may result in the formation of platelet microthrombi and micro-vascular damage.
Gemcitabine is a deoxycytidine analog anti-metabolite that is activated by deoxycytidine kinase because of intra-cellular phosphorylation and inhibits DNA polymerase, resulting in apoptosis of cells.1 The pathogenesis of gemcitabine-induced TMA (GiTMA) is very speculative and remains unexplained. One of the mechanisms might be endothelial damage with consequent clotting system activation as explained by a higher level of nitrate.2 Endothelium injury releases large amounts of von Willebrand factor (vWF) multimers, leading to fibrin deposition and platelet aggregation.3 Because of the expression of CD36 (thrombospondin receptor) on the endothelium of renal and cerebral micro-vasculature, these vessels are more involved compared to the hepatic and pulmonary micro-vasculature.4 Gemcitabine also induces the formation of antibodies against the ADAMTS13 enzyme, which along with elevated levels of vWF multimers results in worsening of micro-vascular thrombosis.4 The patient often presents with dyspnea secondary to non-cardiogenic pulmonary edema, new onset hypertension, or exacerbation of underlying hypertension.5
The patient presented with nephrotic syndrome (NS) associated with renal dysfunction, hemolytic anemia, and thrombocytopenia in this case. There are few case reports of GiTMA presenting as NS.6-8 Severe endothelial dysfunction during the acute phase of glomerular TMA might have led to podocyte loss. In the present biopsy also, there are swollen endothelial cells and electron-dense deposits indicating severe endothelial cell injury.
This patient had developed atypical PRES about 3 weeks after stopping gemcitabine. Discontinuation of the offending drug such as gemcitabine, anti-hypertensive therapy, plasma exchange, and dialysis have been suggested as treatments for GiTMA.5 Our patient was not given any plasma exchange as the role of plasma exchange in the management of chemotherapy-induced TMA is limited, and 50% of patients still progress to end-stage renal disease (ESRD).5 Few case reports have demonstrated remarkable improvement in renal functions in GiTMA after the use of eculizumab. Although this patient did not achieve baseline renal functions, with conservative management, he became stable with improvement in proteinuria.
The onset of nephrotic syndrome with renal dysfunction was followed by a seizure episode diagnosed to be atypical PRES in this case. PRES is a clinical-radiographic syndrome that is hypothesized to be related to disordered cerebral auto-regulation and endothelial dysfunction. The individual may present with headaches, vision changes, confusion, and seizures. Neuro-imaging is typically consistent with vasogenic edema in the subcortical white matter predominantly localized to the posterior cerebral hemispheres but can be found in gray matter or other lobes of the brain. If left untreated, permanent significant neurological impairment or death can occur. There are multiple case reports which described PRES in the context of combination therapy with gemcitabine and a platinum agent.9 There is the possibility that gemcitabine can cross the blood-brain barrier, which may lead to cerebral dysfunction related to PRES. It is also speculated that it may be caused by cerebral vascular endothelial dysfunction because of hypoxia or metabolic abnormality from the chemotherapy. The primary focus in the treatment of PRES is to identify and treat the precipitating factors such as uncontrolled hypertension, vasculitis, auto-immune disease, or discontinuing inciting medications such as calcineurin inhibitors, chemotherapeutic agents, and so on.7-9 In this case report, the patient has clinical and radiological improvement with adequate control of blood pressure and discontinuation of further gemcitabine dose. Anti-epileptics are effectively utilized in patients who exhibit seizure-like episodes. Until now, it is also unclear whether the dosing or frequency of gemcitabine plays a role in developing PRES. It should be considered as an idiosyncratic reaction that can occur at any time after the medication. There is currently no evidence of screening, preventive recommendations, and steroid treatment for chemotherapy-induced PRES. The available works of literature related to NS and PRES reported after gemcitabine monotherapy are depicted in Table 1.6-12
| Authors | Malignancy | PRES or NS | Timing of PRES/NS after the last dose of chemotherapy | Management |
|---|---|---|---|---|
| Russell et al.10 | Stage IV NSCLC and MM | PRES | 3 days | Discontinuation of drug |
| Marrone et al.11 | Stage II Pancreatic | PRES | 1 day | Discontinuation of drug + Phenytoin |
| Truong et al.12 | Stage IV breast | PRES | 5 days | Discontinuation of drug |
| Schaub JR et al.9 | Invasive ductal carcinoma Breast | PRES | 3 weeks after the third cycle | Discontinuation of drug + Ventilatory support + Anti-epileptic drugs |
| Katagiri D et al.8 | Advanced pancreatic carcinoma | TMA with NS | After receiving 11,000 mg gemcitabine (over 8 months) | Supportive treatment + Discontinuation of drug |
| Lee HW et al.6 | Advanced pancreatic carcinoma | TMA with NS | 26,250 mg gemcitabine (over 7 months) | Plasmapheresis + Supportive treatment + Discontinuation of drug |
| Yamada Y et al.7 | Gall bladder carcinoma | TMA with NS | Dose not specified (over 7 months) | Fresh frozen plasma + supportive treatment + Discontinuation of drug |
| Present case | Moderately differentiated periampullary adenocarcinoma | PRES + TMA with NS | After the third cycle (Total dose – 6500 mg gemcitabine) – 3 weeks of last dose | Supportive treatment+discontinuation of drug |
This case uniquely stands out as the patient developed nephrotic syndrome followed by PRES secondary to drug-induced TMA. This case also stresses the importance of continued consideration for the development of TMA manifesting as NS with renal dysfunction and PRES syndrome. The relationship between TMA and PRES is unknown.
Conclusion
Drug-induced systemic TMA manifesting as renal dysfunction with nephrotic syndrome and PRES is rare. This case stresses the importance of early drug discontinuation as the preferred therapy for drug-induced TMA. The roles of plasma exchange, monoclonal antibodies, and other immuno-modulatory drugs are still not clear.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Thrombotic microangiopathy with renal failure in two patients undergoing gemcitabine chemotherapy. Am J Nephrol. 1999;19:590-3.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer chemotherapy-related thrombotic thrombocytopenic purpura: Biological evidence of increased nitric oxide production. Mayo Clin Proc. 1999;74:570-4.
- [CrossRef] [PubMed] [Google Scholar]
- Haemolytic uraemic syndrome associated with bleomycin, epirubicin and cisplatin chemotherapy-a case report and review of the literature. Acta oncologica (Stockholm Sweden). 1998;37:107-9.
- [CrossRef] [PubMed] [Google Scholar]
- Gemcitabine-induced thrombotic microangiopathies: A systematic review. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association-European Renal Association. 2006;21:3038-45. ed. England
- [CrossRef] [PubMed] [Google Scholar]
- The use of eculizumab in gemcitabine-induced thrombotic microangiopathy. BMC Nephrol. 2018;19:9. doi: 10.1186/s12882-018-0812-x
- [CrossRef] [PubMed] [Google Scholar]
- Gemcitabine-induced hemolytic uremic syndrome in pancreatic cancer: A case report and review of the literature. Gut Liver. 2014;8:109-12.
- [CrossRef] [PubMed] [Google Scholar]
- Gemcitabine-induced hemolytic uremic syndrome mimicking scleroderma renal crisis presenting with Raynaud's phenomenon, positive antinuclear antibodies, and hypertensive emergency. Intern Med. 2014;53:445-8.
- [CrossRef] [PubMed] [Google Scholar]
- Gemcitabine induced thrombotic microangiopathy with nephrotic syndrome. CEN Case Rep. 2018;7:217-20.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed gemcitabine-induced posterior reversible encephalopathy syndrome. Am J Med Sci. 2021;361:795-8.
- [CrossRef] [PubMed] [Google Scholar]
- Gemcitabine-associated posterior reversible encephalopathy syndrome: MR imaging and MR spectroscopy findings. Magn Reson Imaging. 2001;19:129-32.
- [CrossRef] [PubMed] [Google Scholar]
- Gemcitabine monotherapy associated with posterior reversible encephalopathy syndrome. Case Rep Oncol. 2011;4:82-7.
- [CrossRef] [PubMed] [Google Scholar]
- Gemcitabine associated with posterior reversible encephalopathy syndrome (PRES): A case report and review of the literature. Clin Adv Hematol Oncol. 2012;10:611-3.
- [Google Scholar]







