Translate this page into:
Neutralizing Anti-SARS-CoV-2 Antibody Response to COVID-19 Vaccines—ChAdOx1-nCoV-19 and BBV152 Among Hemodialysis Patients
Corresponding author: Deepak Kumar Selvanathan, Department of Nephrology, Madras Medical Mission, Mogappair, Chennai, India. Email: dee170388@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Selvanathan DK, Parthasarathy R, Rohit A, Venkatramanan S, D’souza C. Neutralizing Anti-SARS-CoV-2 Antibody Response to COVID-19 Vaccines—ChAdOx1-nCoV-19 and BBV152 Among Hemodialysis Patients. Indian J Nephrol. 2025;35:82-4. doi: 10.25259/IJN_30_2024
Dear Editor,
Patients with end-stage renal kidney disease (ESRD) receiving hemodialysis are at an increased risk of morbidity and mortality with SARS-CoV-2 infection.1 India has seen multiple waves of SARS-CoV-2 infection, with the second wave being more intense having a higher mortality of 18.8 % to 32.7% among the Indian dialysis cohort.2,3 After the COVID vaccine rollout in early January 2021, India has successfully managed to fully vaccinate its population till date. Two vaccines, ChAdOx1-nCoV-19 (Oxford-AstraZeneca, Cambridge, UK) an Adenovirus vector with the spike protein and BBV152 (COVAXIN; Bharat Biotech, Hyderabad, Telangana, India4), a whole cell inactivated vaccine with alhydroxiquim II as adjuvant were first approved by the Drug Controller General of India for use in India. We present a prospective single-center data on neutralizing antibody response to ChAdox1-nCoV-19 and BBV152 vaccines in the maintenance haemodialysis (MHD) patients. Institutional review board approval was obtained.
We prospectively analyzed 149 MHD patients, among which 126 were vaccinated with either one or two doses of ChAdOx1-nCoV-19 and BBV152 between March 2021 and August 2021 [Figure 1]. The dose, duration between the doses and type of vaccine was determined by then prevailing local government guidelines and availability. The choice of the vaccine was that of the patient. Demographic and clinical characteristics were collected from hospital records. The serum antibody titers were done at least 15 weeks or longer of either dose. Titers (Neutralizing antibody titers, miniVidas bioMerieux, France) more than 20.66 binding antibody units were considered as positive humoral response as per WHO recommendations. The cohort were grouped and analyzed as responders and nonresponders. Data on patients developing COVID-19 infection before and after vaccination were collected. Statistical analysis was done with SPSS, IBM v.23, USA.
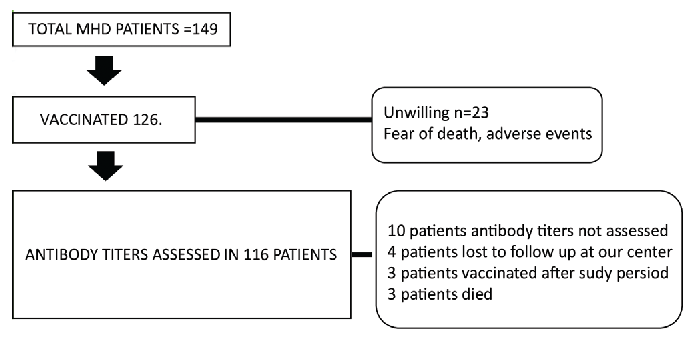
- Flow of study cohort.
Among 149 MHD patients, 23 (15.4%) were unwilling to vaccinate mainly due to fear of adverse events despite appropriate education by the healthcare workers. Only 116 patients (92%) consented to serum antibody testing. In this cohort of 116, 97 (83.6%) were administered ChAdOx1-nCoV19 and 19 (16.3%) received BBV152. Table 1 depicts the baseline characteristics of patients receiving vaccination. The mean age of the cohort was 58.49 ± 12.44 years, with a male predominance (60.3%). The two groups had no significant difference in the comorbidity profile and dialysis vintage. Mean time interval between doses was 69.9 ± 38.1 days and 72.5 ± 45.7 days in patients who received ChAdOx1-nCoV-19 and BBV152, respectively. The median binding antibody units was 246.6 and 93.5 in the ChAdOx1 nCov-19 and BBV152 groups (p = 0.12). Before vaccination, 3.7% and 5.2% of the patients in the ChAdOx1 nCov-19 and BBV152 had contracted COVID-19 infection in the first wave. The main adverse events reported were low-grade fever (17.2%), pain at injection site (8.6%), and breathlessness (1.7%). One patient had Arterio-venous graft thrombosis leading to access failure 2 weeks following ChAdOx1-nCoV-19 vaccination.
| n = 126 |
ChAdOx1-nCoV-19 (n = 107) |
BBV152 (n = 19) |
P value |
|---|---|---|---|
| Age in years* | 57.96 ± 13.6 | 58.84 ± 11.3 | 0.81 |
| Males (%) | 66 (61.6) | 10 (52.6) | 0.32 |
| Diabetes mellitus (%) | 50 (46.7) | 10 (52.6) | 0.80 |
| Hypertension (%) | 101 (94.3) | 18 (94.7) | 0.72 |
| Coronary artery disease (%) | 25 (23.3) | 3 (15.7) | 0.37 |
| Dialysis vintage (in months) ** | 13 (6 - 41) | 32 ( 24 - 48) | 0.18 |
| AV fistula (%) | 81 (75.7) | 15 (78.9) | 0.50 |
| Two doses (%) | 77 (71.9) | 12 (63.1) | 0.58 |
| Time interval between first and second dose of vaccine (in days)* | 69.9 ± 38.1 | 72.5 ± 45.7 | 0.84 |
| Binding antibody units (per ml)** | 246.6 (27.8 – 595.6) | 93.5 ( 4.3 – 321.2) | 0.12 |
| Time to seropositivity in days* | 112.3 ± 52.1 | 121.6 ± 48.66 | 0.94 |
| Malignancy (%) | 2 (1.8) | - | 0.71 |
| Immunosuppression (%) | 8 (7.5) | 1 (5.2) | 0.59 |
| Tuberculosis (%) | 9 (8.4) | - | - |
| Prior kidney transplant (%) | 6 (5.6) | - | - |
| Covid-19 infection pre-vaccination | 4 (3.7) | 1 (5.2) | 0.59 |
| Covid-19 infection post-vaccination | 10 (9.3) | 3 (15.7) | 0.53 |
Of the 116 patients, 88 patients were vaccine responders. Laboratory parameters and the type of vaccine were not significantly different in the responder versus nonresponder groups. The median time to seropositivity after a single dose was 32.5 (30–103) days, and after two doses were 99 (58–122) days. The mean time interval between the first and the second dose was 76 ± 40.2 days in responders which was significantly higher when compared to 53.79 ± 26.8 days in the nonresponders (p = 0.00). Prior COVID-19 infection was seen in 10 patients among the responders but none of the nonresponders.
Of the 25 patients who developed COVID-19 infection in our cohort, 13 (52%) were post-vaccination. Of these 13 patients, 10 had received ChAdOx1-nCoV-19 and 3 received BBV152. Ten (76.9%) completed two doses and the median time to COVID-19 infection was 23.5 days (IQR 16.25–57.5). Symptoms, co-morbidities, dialysis vintage, and vaccine type did not significantly differ among the vaccinated and unvaccinated group. Hospitalization was required in three versus five patients in the unvaccinated and vaccinated groups, respectively [Supplementary Table 1]. Oxygen requirement was lesser among those who contracted COVID-19 infection after vaccination (30.7% vs 75%, p<0.05). Mortality was 8.3% in both the groups. Supplementary Table 2 depicts the detailed COVID-19 infection characteristics among the vaccinated and unvaccinated MHD patients.
Dialysis patients are immunocompromised, resulting in an impaired response to vaccinations with additional risk factors for low antibody response.5 This single-center study from India highlights the response to ChAdOx1-nCoV-19 and BBV152 vaccines among the MHD cohort. This study shows a modest neutralizing antibody response to the spike protein to both of the vaccines with no significant adverse events. COVID-19 infection did not significantly differ in the vaccinated and unvaccinated groups though oxygen requirement was significantly lower among those vaccinated.
Our data demonstrated that both ChAdOx1-nCoV19 and BBV152 elicited a substantial response among the dialysis cohort. Our cohort reported humoral response in 75.8% of the patients with either vaccine, with no difference with respect to the type of vaccination. Fernando and Govindan,6 in their report on 38 Indian hemodialysis patients showed 92.1% seropositivity with high titers. They used IgG anti-spike protein to assess the humoral response. A single dose of either ChAdOx1-nCoV-19 or BBV152 vaccines elicited antibody response in 91.7 % patients, whereas two doses elicited antibody titers in 95.2%. This is in slight contrast to our study where positive antibody response was seen in 78.5% patients. This difference could be attributed to the testing time interval, which was longer in the present study, and the possible differences in the testing kits.
In a study by Anand et al.S1 in the hemodialysis cohort, antibody response was done at three time intervals of less than 14 days, more than 14 days after the first and second doses of mRNA and Johnson and Johnson vaccines. More than 20% of the patients demonstrated an attenuated vaccine response. Though median IgG titers were higher among patients with prior COVID-19 infection, attenuated vaccine response was not different between the two groups. Patients with COVID-19 infection had higher anti-SARS-CoV2 spike protein response to mRNA vaccine in the study by Paal et al.5 with good antibody response (96%) compared to the nondialysis cohort. In the present study, the nonresponders did not have prior SARS-COV-2 infection. Again the timeline of antibody measurement was longer in our cohort than the mRNA and the inactivated vaccine.
In an analysis of multiple studies done on humoral and T-cell responses, majority showed good response in this high-risk cohort of patients. Previous COVID-19 infection again was one of the main factors influencing seroconversion. None of the studies included BBV152 which is unique to the Indian cohort. Many studies have shown that COVID-19 infection with a single dose of vaccination provides a booster-like effect correlating with our study that showed that infection with vaccination resulted in responders rather than nonresponders thus helping individuals mount immunity and may act as a booster dose to the immune system along with the vaccination.
COVID-19 infection was similar between the vaccinated and unvaccinated in the present study. This may be due to the initial vaccine drive being concurrent with the second wave of infection and the vaccine roll out being staggered across age groups. Hospitalization rates were similar as the quarantine policies for high-risk patients were determined by local laws. Overall, the morbidity, oxygen requirement, and need for antivirals were lower in the vaccinated group.
This study had limitations. Antibody estimation prior to first dose of vaccination is not available rendering it difficult to estimate the actual immunity conferred by the infection itself versus the vaccine alone. Serial monitoring of antibody titers at fixed intervals between the first and second dose could not be done. T-cell responses which would contribute importantly to the longevity of immunity against SARS-CoV-2 were not measured.
Conflicts of interest
There are no conflicts of interest.
References
- Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney int. 2020;98:1530-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The high mortality and impact of vaccination on COVID-19 in haemodialysis population in India DURING the Second wave. Kidney Int rep. 2021;6:2731.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A clinical study on the changing dynamics of disease severity, management strategies and outcomes of COVID-19 in patients requiring haemodialysis. J Nephrol. 2021;14:2234-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antibody response to mRNA SARS-CoV-2 vaccines in haemodialysis patients. Clin Kidney J. 2021;14:2234-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neutralizing SARS-CoV-2 antibody response and protective effect of 2 doses of ChAdOx1 nCoV-19 and BBV152 vaccines in haemodialysis patients: A preliminary Report. Kidney Int Rep. 2021;6:2521-2.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






