Translate this page into:
Not Just Small Adults: Considerations for Pediatric Chronic Kidney Disease
Corresponding author: Nivedita Kamath, Department of Pediatric Nephrology, St. John’s Medical College Hospital, Bangalore, Karnataka, India. E-mail: n_shenoy25@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Gerber A, Kamath N. Not Just Small Adults: Considerations for Pediatric Chronic Kidney Disease. Indian J Nephrol. doi: 10.25259/IJN_77_2024
Abstract
Chronic kidney disease (CKD), including pediatric CKD, is a global public health concern. Pediatric CKD has lasting effects into adulthood. In this review, we focus on the etiology of pediatric CKD and unique aspects that should be considered in treating a child with CKD, such as ensuring adequate nutrition and assessing growth hormone axis dysregulation. We review risk factors for CKD progression and how clinical surveillance can be used to address modifiable factors. We address the issues of accurate glomerular filtration rate (GFR) estimation, cardiovascular disease, immunization, neurodevelopment, and planned transition to adult care. We also cover kidney failure preparation and global CKD care disparities affecting children worldwide.
Keywords
Chronic kidney disease
Children
Nutrition
Growth hormone
Disparities
Introduction
Chronic kidney disease (CKD) is a global public health concern.1 In adults, the prevalence, morbidity, and mortality of CKD are rising.2 For children, the global prevalence of CKD is estimated around 15-96 cases per million, though epidemiologic data for pediatric CKD are scarce.3,4 Although CKD affects a relatively small proportion of children compared to adults globally, the number of children affected is growing in certain regions.5–7 Pediatric CKD has distinct features that impact patients lifelong.8 This review highlights unique aspects of CKD in children, including diagnosis of CKD, common etiologies and risk factors for progression, secondary growth impairment, neurocognitive impacts, preparation for kidney failure (KF), eventual transition of care, and leading causes of mortality. We also discuss the global disparities in accessing life-saving kidney replacement therapy (KRT) for children with KF.
CKD diagnosis and risk factors in children
Measuring estimated glomerular filtration rate (eGFR)
CKD is characterized by gradual loss of kidney function over time. Early diagnosis and prompt treatment are crucial for delaying or preventing sequelae related to CKD, but determining the degree of kidney dysfunction in children can be challenging.9 Conventional CKD staging relies on body surface area (BSA) adjusted eGFR.10–12 From 0 to 2 years old, renal blood flow increases in response to higher mean arterial pressure and decreased renal vascular resistance.13 Consequently, at two years old, children have BSA-adjusted eGFR similar to healthy adults, but infants and toddlers have lower BSA-adjusted eGFR before two years old.13 Thus, age-specific GFR values must be utilized instead of conventional CKD staging for children younger than two years. For infants and young children, the severity of CKD sequelae can also help classify the degree of CKD.14
Creatinine-based eGFR measurements used for adults are less accurate for children.15,16 A more accurate approach is to use a combination of creatinine and cystatin C. Cystatin C is a more expensive test than creatinine, but, unlike creatinine, it is unaffected by muscle mass.12,14,15 The Schwartz Formula (2009) is a widely accepted creatinine-only method of estimating GFR in children: multiply height (cm) by 0.413 and divide by serum creatinine (mg/dL).16 The Chronic Kidney Disease in Children Under 25 (CKiD U25) calculator is increasingly preferred because it exhibits less bias across a more comprehensive age range than the Schwartz equation and has options for calculating eGFR based on creatinine, cystatin C, or both.17–19
More population diversity in deriving GFR estimating equations is required to establish global validity.20 North American and European registries have disproportionately enrolled children with European ancestry, and further work is needed to examine the performance of eGFR equations in populations with other ancestral histories, including South Asians.21,22
The Kidney Disease Improving Global Outcomes (KDIGO) guidelines for adults use a degree of albuminuria and eGFR to stage CKD.10 However, albuminuria is not as functional for CKD staging in pediatrics due to the predominance of non-glomerular kidney diseases.23 For children with non-glomerular etiologies of CKD, albuminuria is only seen with kidney scarring in later CKD stages.24 Although serum markers are primarily used for pediatric CKD staging, proteinuria can be a valuable marker for prognostication.25 For children with CKD, persistent proteinuria (urine protein-to-creatinine ratio of >2 for those with non-glomerular etiologies and >0.5 for those with glomerular etiologies) predicts significant CKD progression.26
Screening for pediatric CKD
Some countries, including Korea, Taiwan, and Japan, have national urinary screening programs for early detection of pediatric CKD.27 Screening remains controversial in other countries because of the low incidence of pediatric CKD and the commonality of false positive urine dipstick results. In the United States (U.S.), 8,954 children received screening urine dipsticks, of which 1,264 had abnormal results. Only 11 of the 1,264 (0.1% of the total sample) were diagnosed with CKD.28 The screening program costs $2997.50 per case of diagnosed CKD.28 Thus, the American Academy of Pediatrics does not recommend routine urinary dipstick screening for children.29
Currently, India does not have national guidelines for routine pediatric CKD screening. More information is needed on the prevalence of pediatric CKD in India to determine the cost-benefit of a nationwide screening program.30 Even in countries where routine screening for pediatric CKD is not recommended, there may be specific higher-risk subgroups, such as premature infants, who would benefit from screening.31
Etiology of pediatric CKD
Only a few large population-based studies of pediatric CKD have been conducted, in part due to the asymptomatic nature of early-stage CKD.23 Existing data demonstrate that the most common cause of CKD in children is congenital anomalies of the kidney and urinary tract (CAKUT), accounting for 50-65% of children with CKD.32,33 Consequently, although albuminuria is a sensitive marker for CKD severity in adults, it is a less sensitive marker for children, in whom non-glomerular causes of CKD are more common.14 CAKUT can be an isolated finding or part of a genetic syndrome, and prenatal ultrasounds are key for early diagnosis.9,18,32,34
Other common etiologies of pediatric CKD are obstructive uropathy, steroid resistant nephrotic syndrome (e.g., focal segmental glomerulosclerosis) and chronic glomerulonephritis, which together account for 20% of CKD in children. Alport syndrome, cystinosis, and post-acute kidney injury together account for 15%.9,34 Monogenic causes are found in 10-60% of children with CKD due to CAKUT or glomerulonephritis.25,26 Certain risk alleles have also been identified. For example, children with sickle cell disease and APOL1 risk variants are reported to develop a more rapid decline in eGFR than those without.34,35 These findings emphasize the utility of targeted genetic testing for pediatric patients with CKD, both for diagnostics and to guide clinical management.36–38
Risk factors for CKD progression in children
Like the adult population, hypertension, proteinuria, and obesity are independent risk factors for CKD progression in the pediatric population [Figure 1].39 The ESCAPE trial showed that intensified BP control for children with CKD provided a 35% relative risk reduction in CKD progression compared to more permissive BP management.40 The CKiD study showed that the CKD stage stratified by eGFR and proteinuria characterizes the timeline of progression to kidney failure, as seen in adults.33 In children, non-glomerular disease is the most common cause of CKD and has a 43% slower progression when compared to glomerular disease.41
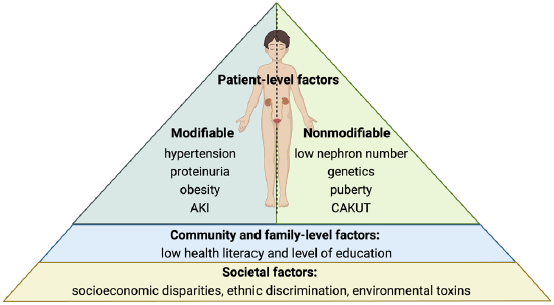
- Risk factors for CKD progression. Created with BioRender.com. AKI: acute kidney injury; CAKUT: congenital abnormalities of the kidney and urinary tract; CKD: chronic kidney disease
Obesity can lead to kidney injury from increased intraglomerular pressure.12 The International Pediatric Peritoneal Dialysis Network found that 19.7% of children worldwide were overweight or obese at the start of chronic peritoneal dialysis (PD), underscoring the need for early establishment of habits such as energy intake moderation (without compromising nutrition) and regular physical activity.42
Low birth weight and prematurity are also risk factors for CKD progression. Each human kidney has 1 million nephrons on average, though that number varies widely.43 Most nephrons are formed during the third trimester of pregnancy, and low birth weight and prematurity are linked to low nephron number. For babies born premature, nephrogenesis continues in the extrauterine environment for a limited time, but extrauterine nephrogenesis is associated with fewer layers of nephrons and nephron maldevelopment.44–46 Decreased nephron number and nephron maldevelopment lead to increased CKD risk both in childhood and later in life, supporting the concept of “fetal programming” even in adult CKD.39,47
Urologic problems also impact CKD progression.14 The kidney and urinary tract develop through reciprocal interactions between the ureteric bud, which forms the urinary collecting system, and the metanephric mesenchyme, which forms nephrons.48 Many young children with urinary tract abnormalities have normal eGFR. However, they may still have microscopic kidney dysplasia because of these complex two-way signaling pathways through which the kidney and urinary tract develop.35
Urologic abnormalities—such as bladder dysfunction commonly seen in boys with posterior urethral valves, neurogenic bladder, and vesicoureteral reflux (VUR)—predispose children to recurrent urinary tract infections (UTIs) and kidney scarring.49 Recurrent UTIs can increase the risk of CKD progression, and antibiotic prophylaxis may be indicated in high-grade VUR.50–53 To optimize bladder health, constipation should be prevented and treated. Strategies such as timed and double voiding are also helpful in preventing urine withholding.54 Understanding how to care for patients with CAKUT is relevant for pediatric and adult providers since half of patients with CAKUT who progress to KF do so after the first three decades of life.14,36,55
Rapid periods of growth, including puberty, are critical windows to surveil CKD progression. eGFR declines 10 times faster after the pubertal growth spurt.56 Regular clinical surveillance is also an opportunity to reinforce kidney protective habits that can mitigate CKD progression, including preventing acute kidney injury (AKI) from nephrotoxic medications and dehydration. Similar to adults, AKI is a risk factor for the progression of CKD in children.57
Crucially, social inequities and disparities influence the risk of pediatric CKD progression worldwide.14,58,59 In the CKiD cohort, there was an association between higher parental health literacy and slower progression of CKD.60 Faster GFR decline in black children compared to white children in the U.S. is also influenced in part by non-biological disparities, such as racial disparities in socioeconomic and parental education status, exposure to environmental risk factors such as air pollution and water toxins, and experiences with structural racism.14,58
Care of a child with CKD
This section covers complications of CKD in children, many of which are similar to those seen in adults. We emphasize issues that pose unique challenges for children, such as growth failure, neurocognitive outcomes, preparation for kidney failure, and the transition of healthcare between adolescence and young adulthood [Figure 2].
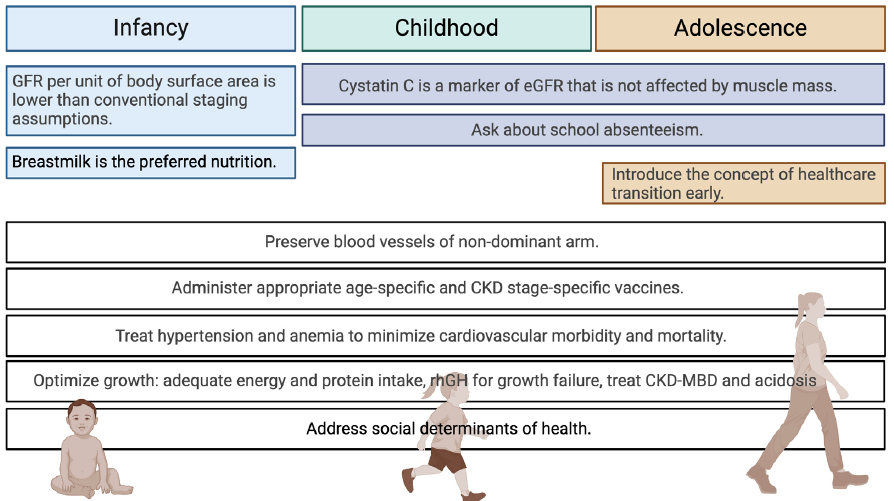
- Pearls for pediatric CKD management. Created with BioRender.com. GFR: glomerular filtration rate; CKD: chronic kidney disease; MBD: mineral bone disease, eGFR: estimated glomerular filtration rate
Growth failure in children with CKD
Childhood is a critical time of skeletal growth in size and strength, and bone mass achieved in childhood and adolescence determines bone health over the entire lifespan.9,61,62 Children can have impaired growth at any stage of CKD, though growth is increasingly impaired as eGFR declines.63–65 The 2006 North American Pediatric Transplant Cooperative Study (NAPRTCS) reported that one-third of children had a height below the third percentile at the time of study enrollment.66 Earlier age of CKD diagnosis is associated with a greater risk of growth impairment.65,67
Short stature is important to address because it is perhaps the most visible sequela of pediatric CKD and can have negative psychosocial impacts on the child.63,68 Moreover, short stature is associated with increased morbidity—including decreased school attendance and increased hospital days and mortality in children starting dialysis.69,70
Multiple factors must be addressed to optimize growth in children with progressive CKD, including metabolic acidosis, malnutrition, mineral and bone disorders (CKD-MBD), anemia, and dysregulation of growth hormone metabolism.
Malnutrition
Although the prevalence of overnutrition in children with CKD is rising, children with CKD, especially in limited resource settings, remain at risk for malnutrition and protein-energy wasting, leading to poor growth.42,71 Children with progressive CKD frequently experience vomiting, poor appetite, and reduced taste sensation which contributes to malnutrition.63 Food insecurity, defined as the limited or uncertain availability of nutritionally adequate and safe foods, also contributes to undernutrition in pediatric patients with CKD.72,73 A single-center study in the U.S. found that 64% of children on dialysis experienced food insecurity.74
The 2020 Pediatric Renal Nutrition Taskforce recommendations state that energy intake for children with CKD should match with healthy children of the same chronological age, adjusted toward the higher end of the range for those with growth impairment.75 The 2008 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend that children with CKD stages 2-5 should receive at least 100% of the daily recommended intake (DRI) of protein.76 There is no evidence that dietary protein restriction is protective against CKD progression in children, and a low-protein diet may compromise growth.76,77 However, if needed, protein intake can be safely restricted to the lower end of the DRI range to reduce nitrogenous waste product accumulation and lower dietary phosphorous intake.78
Nutrition is essential in optimizing growth over the first two years of life.63,67,75 While oral feeding is preferred, children who cannot consume adequate calories or tolerate their medications by mouth may need a gastrostomy tube. If a family is planning to pursue peritoneal dialysis (PD) for their child with kidney failure and a gastrostomy tube is indicated for nutrition, the gastrostomy tube should be placed either before or at the same time as PD catheter placement to reduce the risk of peritonitis.79
Breast milk is preferred for an infant with CKD because of its low renal solute load. Whey-dominant infant formulas should be used when breastmilk is unavailable because they mimic breastmilk’s protein and electrolyte profile closer than casein-based formulas.75 Breastmilk and infant formulas can be fortified to provide adequate nutrition in fluid restriction or when more nutrient-dense feeds are indicated.75,76
Adequate nutrition can be particularly challenging for children with tubular disorders that result in electrolyte losses. Electrolyte supplementation can cause abdominal pain and diarrhea, and the sheer volume of supplementation required to maintain acceptable electrolyte levels can be difficult for a small child to meet. Helpful strategies for these patients include consideration of gastrostomy tube placement to facilitate medication administration, alternative forms of electrolyte supplementation, such as liposomal magnesium supplementation, that are better tolerated, and thorough nutritional counseling.80 All children with CKD benefit from having a kidney nutritionist or dietician as part of their healthcare team.75,76
Dysregulation of growth hormone metabolism
While malnutrition is the principal driver of poor growth in young children with CKD, dysregulation of growth hormone (GH) and insulin growth factor-1 (IGF-1) metabolism is the main contributor after early childhood.9,67 CKD is a state of relative GH and IGF-1 resistance and insensitivity.81 Long-term exposure to high-dose glucocorticoids, which are indicated in some forms of chronic glomerular disease, has been shown to suppress pituitary GH release further and downregulate hepatic GH receptors as well as induce IGF inhibitors.63
Recombinant human GH (rhGH) improves growth in children with CKD with impaired growth and has relatively few side effects.9,63,67 A cochrane meta-analysis concluded that children who receive rhGH at 25 IU/m2 weekly for 1 year significantly increase height, with decreasing effect in subsequent years.82 Although the height increase with rhGH is seen regardless of pubertal status or CKD stage, prepubertal children have more significant height gains than postpubertal children, and children with CKD stage 3 or 4 have more significant height gains than those with CKD stage 5.63,82 rhGH treatment should be considered in children with CKD stage 3-5 when metabolic acidosis and malnutrition, including inadequate sodium balance, have been sufficiently addressed.23,83
Although no randomized controlled trials have evaluated the association between rhGH administration in childhood CKD and final adult height, a case-control study concluded that children treated with GH had sustained catch-up growth. In contrast, the control group had progressive growth impairment.84 Unfortunately, despite evidence of its benefits in children with CKD, rhGH is still not accessible for many around the world due to its high cost.83
Thyroid dysfunction
Increased prevalence of hypothyroidism has been reported in children with CKD stage 4 and 5.85 Treatment of overt hypothyroidism improves growth in children with CKD. Some studies have shown that even treatment of persistent subclinical hypothyroidism can slow GFR decline.86
Pubertal delay
Delayed puberty may lead to reduced linear height in adolescents with CKD. CKD interferes with the neurohypophyseal reproductive axis. As a result, adolescents with late-stage CKD lack pulsatile secretion of luteinizing hormone and gonadotropin-releasing hormone.87 Pubertal delay is most pronounced in children requiring long-term dialysis and those who have a long-term requirement for high-dose glucocorticoids. In adolescents with ESKD, early kidney transplantation and limiting post-transplant glucocorticoid exposure to 6 months or less helps facilitate normal pubertal development.88
CKD - Mineral bone disease
Clinical, biochemical, and radiological features of mineral bone disease are commonly seen in children with CKD. Dysregulation of mineral metabolism in children with CKD is a risk factor for cardiovascular disease and results in similar complications to those seen in adults: extraskeletal calcification, fractures, bone pain, and avascular necrosis. Children who are experiencing CKD-MBD during critical growth windows are also susceptible to unique complications such as skeletal deformities and short stature.89
Strategies for preventing and managing CKD-MBD in children are similar to those for adults. Age-appropriate calcium and phosphate goals should be targeted through dietary restriction and phosphate binders. For children, calcium-based phosphate binders are the first line for hyperphosphatemia management in the absence of hypercalcemia and accelerated extraskeletal calcifications.89,90
A deficiency of 25-hydroxyvitamin D should be corrected, and active vitamin D analogs should be considered in patients with late-stage CKD with hyperparathyroidism. Active Vitamin D analogs are also essential in treating hypocalcemia related to phosphate retention from CKD and 1,25-dihydroxy vitamin D deficiency. Parathyroid hormone should be maintained at near normal levels in children with CKD stage 2-5 and at 2-3 times the upper limit of normal for those on dialysis.9,90
Tight management of parathyroid hormone, calcium, and phosphorous levels is critical for healthy bone remodeling and somatic growth in children with CKD.9,63 Serum biomarkers of MBD must be evaluated at regular intervals and interpreted based on age-specific reference ranges. Metabolic acidosis should be concurrently corrected with oral alkali administration.90
Anemia of CKD
Anemia is a common complication of CKD in children and is associated with increased risk for hospitalization, adverse cardiovascular outcomes such as left ventricular hypertrophy (LVH), and mortality. Age-specific hemoglobin cut-offs must be used to diagnose anemia in children.91
Iron deficiency anemia is the most common form of anemia in childhood and can be treated with oral or intravenous iron, especially if the child is on hemodialysis.92 Children with anemia of CKD can be safely treated with recombinant human erythropoietin (rHuEPO), targeting a hemoglobin level of 11-12 g/dl.62,92 Younger children may require higher doses of rHuEPO than adults, likely due to more non-hematopoietic erythropoietin binding sites in children that reduce overall bioavailability.9,93
Neurocognitive impact
Severe neurocognitive impairment in children due to CKD is uncommon with today’s advances in CKD care, including better nutrition, anemia treatment, and avoidance of aluminum-containing phosphate binders. However, subtle neurocognitive impairment can still be seen in children of all ages with CKD.23 Earlier age of diagnosis, longer duration of disease, later CKD stage, proteinuria, and hypertension are risk factors for neurocognitive impairment. Genomic variants are also implicated.94,95
Children in the preschool age group with mild to moderate CKD demonstrate deficits in attention regulation and social and adaptive behavior. Though the intelligence quotient seems to be preserved in older children and adolescents with CKD, difficulties are still noted in attention regulation and executive function. Academic underachievement is noted in about a third of children with CKD, which can be compounded by CKD-related school absences and environmental factors such as socioeconomic status and maternal education level.96,97
Cardiovascular complications and death
Mortality rates for children with progressive CKD are 30 times higher than rates for age- and sex-matched peers but have been decreasing over time.14,98,99 Cardiovascular disease (CVD) is a leading cause of death in both adults and children with CKD.14,99 While adult deaths from CVD are mostly related to coronary artery disease and congestive heart failure, pediatric deaths from CKD are primarily caused by arrhythmias, valve disease, cardiomyopathy, and cardiac arrest, as summarized by Becherucci et al.9,100,101 LVH, which precipitates systolic and diastolic dysfunction and arrhythmias, is the most common cardiac abnormality in pediatric CKD.9,100 Children with ESKD have a 1000 times higher risk of death from CVD compared to the non-CKD pediatric population.102 Modifiable risk factors for CVD should be closely monitored and managed, including obesity, dyslipidemia, impaired glucose metabolism, anemia, vascular calcification from increased calcium-phosphorous product, and hyperparathyroidism.9
Infection risk and vaccine-preventable illnesses
Infection is another leading cause of death in children with CKD.14 Children with progressive CKD have impaired immune responses and are at increased risk for infections.12 It is imperative that they be appropriately vaccinated. Children with CKD should receive the influenza vaccine annually and adhere to CKD stage-specific and age-specific recommendations for pneumococcal, meningococcal, and hepatitis B vaccines.12,103
Children on hemodialysis are at risk for hepatitis B infection. They should have their antibody titers measured if they have completed the primary vaccination series to determine whether they need reimmunization. Patients with nephrotic syndrome are at risk for invasive Streptococcus pneumoniae infections. They should receive the 23-valent polysaccharide pneumococcal conjugate vaccine after they turn two years old and at least 8 weeks after they receive the 13-valent pneumococcal conjugate vaccine.104 Live-viral vaccines should be avoided in children on immunosuppressive therapies, including those who have received kidney transplants.102,105
Preparing for kidney failure (KF)
While working to preserve kidney function, we simultaneously prepare our patients and their families for what may happen if their CKD progresses to late stages. Successful hemodialysis requires long-term vascular access. Thus, for all children with progressive CKD, the forearm veins of the non-dominant hand should be preserved. Central lines in the subclavian vein and peripherally inserted central catheters should be avoided.106 Anemia management with rHuEPO and iron is key to preventing frequent blood transfusions, which can increase Human Leukocyte Antigen (HLA) sensitization and make it difficult to find a compatible kidney transplant.107
For children with KF, preemptive kidney transplant is the gold standard treatment.108 Before a kidney transplant, children with a history of CAKUT, bladder dysfunction, or recurrent UTIs should have a urologic consultation to determine whether the bladder is safe for transplant or whether a ureterostomy is required.109 When a preemptive transplant is not feasible, the selection of dialysis modality is based on the child’s age, size, comorbidities, caregiver support, contraindications to peritoneal or vascular access, local dialysis expertise, and personal preference.110 Timing of dialysis initiation is also essential since a peritoneal dialysis catheter should be given time to heal and scar down, whereas a hemodialysis catheter can be used immediately.111
Dialysis initiation should be considered at an eGFR of <10 ml/min/1.73m2 or if the child fails medical and nutritional management of uremic symptoms.110 Peritoneal dialysis is the most widely used modality in younger children. It allows for regular school attendance, but this home-based approach may place a more significant care burden on families than hemodialysis.110 Regardless of dialysis modality, families benefit from early and frequent education on catheter care and return precautions for suspected catheter-related infections.
Challenges in KF preparation for infants
Challenges in the management of infants with KF include ethics surrounding if and when to initiate kidney replacement therapy given uncertain survival to transplant and common comorbidities such as neurodevelopmental delay, infections, and cardiorespiratory disease. If an infant is being initiated on peritoneal dialysis, the PD catheter should be placed far away from the diaper area as possible, often in the right upper quadrant, so it is also away from a gastrostomy tube. Volume management is another unique challenge for infants on peritoneal dialysis and hemodialysis because their diet is mainly in the liquid form. Transplantation is often delayed until an infant reaches a weight—around 8-10kg—that makes transplantation surgery technically feasible.112
Transition of care from pediatric to adult medical providers
Strategically planned healthcare transition when adolescents with CKD approach young adulthood is associated with improved health outcomes. Coordinated healthcare transition, as opposed to abrupt transfer of care, is associated with decreased rates of kidney allograft rejection or loss and improved patient-centered outcomes, such as patient satisfaction, optimism, and engagement in their medical care.113
The primary challenge to standardized and widely available healthcare transition programs in nephrology clinics is their perceived time- and resource-intensive nature. However, as outlined in the International Society of Nephrology – International Pediatric Nephrology Association (ISN-IPNA) 2011 consensus statement, this challenge can be overcome with a gradual approach to healthcare transition, starting with simply introducing the concept when patients are 12 years old. From there, preparing for transition can occur throughout adolescence as the patient learns to communicate independently with medical providers and develops skills for disease self-management.113 Transition programs should, at minimum, include a pediatric nephrologist, an adult nephrologist, and nurse coordinators.
Global disparities in CKD care for children
Global disparities directly impact medical care for children with CKD. About 80% of the data on the prevalence of CKD and KF in children is derived from registries in high-income countries. Data from low- and middle-income countries (LMICs) is meager and does not accurately reflect the burden of CKD.3 In low-resource regions, late presentation may obscure the etiology of CKD. One tertiary care center in India reported that about 20% of children were diagnosed with CKD when they presented to the hospital with life-threatening complications.114 The preference for traditional and alternative medications in low-resource regions may also delay appropriate medical care.115
Children from LMICs have a faster decline in GFR than children living in other parts of the world and experience rapid progression to KF.116 The burden of CKD complications such as malnutrition, growth failure, uncontrolled hypertension, and cardiovascular disease is also higher in LMICs.115
Access to dialysis for KF is directly related to the gross national income.3,117 Less than 10% of children in LMICs who require dialysis receive it.3 Few children in LMICs have access to kidney transplantation, and most discontinue treatment and die before transplantation becomes a realistic option.117 In comparison, children in high-income countries with KF have had decreasing mortality rates over the last 30 years due to financially and logistically accessible kidney replacement therapy (KRT), encompassing dialysis and transplant. The pediatric nephrology community is tasked with expanding this access to children across the globe.117
Conclusion
The silent nature of early pediatric CKD necessitates regular clinical surveillance to prevent and promptly address modifiable risk factors related to disease progression. These surveillance efforts are complicated by a lack of awareness surrounding pediatric CKD and difficulties in accurately measuring kidney function in children. Pediatric CKD management should consider the unique considerations of optimizing skeletal growth, brain development, and cardiovascular health; ensuring CKD stage-appropriate and age-appropriate vaccination; and preparing for eventual healthcare transition to adult providers. Although pediatric CKD remains relatively uncommon, its sequelae have a lifelong impact. The contributing biological and non-biological risk factors, including social disparities, must be addressed thoroughly to preserve children’s physical health and quality of life.
Conflicts of interest
There are no conflicts of interest.
References
- An integrated approach towards a public health perspective on chronic kidney disease. Nat Rev Nephrol. 2022;18:131-2.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The global burden of chronic kidney disease. The Lancet. 2020;395:662-4.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of chronic kidney disease in children. Pediatric Nephrology. 2012;27:363-73.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease in children: The global perspective. Pediatric Nephrology. 2007;22:1999-2009.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease in children and adolescents in brunei darussalam. World J Nephrol. 2016;5:213.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease causes and outcomes in children: Perspective from an LMIC setting. PLoS One. 2022;17
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of chronic kidney disease in children: A report from lithuania. Medicina (Lithuania). 2021;57
- [Google Scholar]
- A review of pediatric chronic kidney disease. In: Blood purification. Vol 41. S. Karger AG; 2016. p. :211-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease in children. Clin Kidney J. 2016;9:583-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kidney disease: improving global outcomes chronic kidney disease guideline development work group members. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825-30.
- [CrossRef] [PubMed] [Google Scholar]
- National kidney foundation’s kidney disease outcomes quality initiative clinical practice guidelines for chronic kidney disease in children and adolescents: Evaluation, classification, and stratification. Pediatrics. 2003;111:1416-21.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric chronic kidney disease. Adv Pediatr. 2022;69:123-132.
- [CrossRef] [PubMed] [Google Scholar]
- Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013;3:19-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of pediatric chronic kidney disease/kidney failure: Learning from registries and cohort studies.
- Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832-43.
- [CrossRef] [PubMed] [Google Scholar]
- New Equations to estimate gfr in children with CKD. J Am Soc Nephrol. 2009;20:629-37.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99:948-56.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development of an adaptive clinical web-based prediction tool for kidney replacement therapy in children with chronic kidney disease. Kidney Int. 2023;104:985-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79:268-88.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Self-reported race, Serum creatinine, Cystatin C, and GFR in children and young adults with pediatric kidney diseases: A report from the chronic kidney disease in children (CKiD) study. Am J Kidney Dis. 2022;80:174-85.e1.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact of chronic kidney disease epidemiology collaboration (CKD-EPI) GFR estimating equations on CKD prevalence and classification among asians. Front Med (Lausanne). 2022;9
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease in children. Pediatr Clin North Am. 2022;69:1239-54.
- [CrossRef] [PubMed] [Google Scholar]
- Reflux nephropathy and scarring nephropathy: So close and yet so different. Anales de Pediatría (English Edition). 2022;97:40-7.
- [PubMed] [Google Scholar]
- Proteinuria in children: Evaluation and differential diagnosis. . 2017;95:248-54. www.aafp.org/afp
- [PubMed] [Google Scholar]
- Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: The chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis. 2015;65:878-88.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cost-Effectiveness of school urinary screening for early detection of IgA nephropathy in japan. JAMA Netw Open. 2024;7:E2356412.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Cost-effectiveness analysis of screening urine dipsticks in well-child care. Pediatrics. 2010;125:660-3.
- [CrossRef] [PubMed] [Google Scholar]
- Urinalysis and urine culture. Pediatric care online. Published online September 30, 2020
- Urinary screening in asymptomatic Indian children: A cross sectional epidemiological study. EJIFCC. 2022;33:242-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prematurity and future kidney health: The growing risk of chronic kidney disease. Curr Opin Pediatr. 2018;30:228-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Contributions of the transplant registry: The 2006 annual report of the north american pediatric renal trials and collaborative studies (NAPRTCS) Pediatr Transplant. 2007;11:366-73.
- [CrossRef] [PubMed] [Google Scholar]
- The CKiD study: Overview and summary of findings related to kidney disease progression. Pediatric Nephrology. 2021;36:527-38.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease emerging trends in children and what to do about it. J Natl Med Assoc. 2022;114:S50-S55.
- [CrossRef] [PubMed] [Google Scholar]
- A focus on the association of Apol1 with kidney disease in children. Pediatr Nephrol. 2021;36:777-88.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease in children: An update. Clin Kidney J. 2023;16:1600-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genetic testing in the diagnosis of chronic kidney disease: Recommendations for clinical practice. Nephrol Dial Transplant. 2022;37:239-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical utility of genetic testing in Indian children with kidney diseases. BMC Nephrol. 2023;24
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of pediatric chronic kidney disease/kidney failure: Learning from registries and cohort studies. Pediatr Nephrol. 2022;37:1215-29.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:39-1650.
- [CrossRef] [PubMed] [Google Scholar]
- Estimating time to ESRD in children With CKD. Am J Kidney Dis. 2018;71:783-92.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global variation of nutritional status in children undergoing chronic peritoneal dialysis: A longitudinal study of the international pediatric peritoneal dialysis network. Sci Rep. 2019;9:4886.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The impact of kidney development on the life course: A consensus document for action. Nephron. 2017;136:3-49.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol. 2004;7:17-25.
- [CrossRef] [PubMed] [Google Scholar]
- Kidney growth following preterm birth: Evaluation with renal parenchyma ultrasonography. Pediatr Res. 2023;93:1302-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Short-term gestation, long-term risk: Prematurity and chronic kidney disease. Pediatrics. 2013;131:1168-79.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease and dietary measures to improve outcomes. Pediatr Clin North Am. 2019;66:247-67.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A coordinated progression of progenitor cell states initiates urinary tract development. Nat Commun. 2021;12:2627.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136:e13-e21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evidence-based clinical practice guideline for management of urinary tract infection and primary vesicoureteric reflux. Pediatric Nephrology. 2024;39:1639-68.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Progression of chronic kidney disease in children with vesicoureteral reflux: The north american pediatric renal trials collaborative studies database. J Urol. 2009;182:1678-82.
- [CrossRef] [PubMed] [Google Scholar]
- Pyuria, urinary tract infection and renal outcome in patients with chronic kidney disease stage 3–5. Sci Rep. 2020;10:19460.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Childhood urinary tract infections as a cause of chronic kidney disease. Pediatrics. 2011;128:840-47.
- [CrossRef] [PubMed] [Google Scholar]
- Current strategies to predict and manage sequelae of posterior urethral valves in children. Pediatric Nephrology. 2018;33:1651-61.
- [CrossRef] [PubMed] [Google Scholar]
- Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol. 2013;8:67-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of puberty with changes in GFR in children with CKD. Am J Kidney Dis. 2022;79:131-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury in childhood: Should we be worried about progression to CKD? Pediatric Nephrology. 2011;26:509-22.
- [CrossRef] [PubMed] [Google Scholar]
- Kidney disease in african american children: Biological and nonbiological disparities. Am J Kidney Dis. 2018;72:S17-21.
- [CrossRef] [PubMed] [Google Scholar]
- Social and economic determinants of pediatric health inequalities: The model of chronic kidney disease. Pediatr Res. 2016;79:159-68.
- [CrossRef] [PubMed] [Google Scholar]
- Parental health literacy and progression of chronic kidney disease in children. Pediatric Nephrology. 2018;33:59-1764.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association between linear growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr. 2017;171
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nutrition and growth in children with chronic kidney disease. Nat Rev Nephrol. 2011;7:615-23.
- [CrossRef] [PubMed] [Google Scholar]
- Nutrition and growth in relation to severity of renal disease in children. Pediatr Nephrol. 2000;15:259-65.
- [CrossRef] [PubMed] [Google Scholar]
- Contributions of the transplant registry: The 2006 annual report of the north american pediatric renal trials and collaborative studies (NAPRTCS) Pediatr Transplant. 2007;11:366-73.
- [CrossRef] [PubMed] [Google Scholar]
- Stature in children with chronic kidney disease: Analysis of NAPRTCS database. Pediatric Nephrology. 2006;21:793-9.
- [CrossRef] [PubMed] [Google Scholar]
- Growth and nutrition in pediatric chronic kidney disease. Front Pediatr. 2018;6
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Psychosocial rehabilitation and satisfaction with life in adults with childhood-onset of end-stage renal disease. Pediatric Nephrology. 2005;20:1288-94.
- [CrossRef] [PubMed] [Google Scholar]
- Anthropometric measures and risk of death in children with end-stage renal disease. Am J Kidney Dis. 2000;36:811-9.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse clinical outcomes associated with short stature at dialysis initiation: A report of the north american pediatric renal transplant cooperative study. Pediatrics. 2002;109:909-13.
- [CrossRef] [PubMed] [Google Scholar]
- malnutrition patterns in children with chronic kidney disease. Life. 2023;13:713.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Core indicators of nutritional state for difficult-to-sample populations. J Nutr. 1990;120:1559-600.
- [CrossRef] [PubMed] [Google Scholar]
- Food insecurity and kidney disease: Symptoms of structural racism. Clin J Am Soc Nephrol. 2021;16:1903-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of food insecurity and acute health care utilization in children with end-stage kidney disease. JAMA Pediatr. 2019;173:1097-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Energy and protein requirements for children with CKD stages 2-5 and on dialysis–clinical practice recommendations from the pediatric renal nutrition taskforce. Pediatric Nephrology. 2020;35:519-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KDOQI clinical practice guideline for nutrition in children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009;53:S11-04.
- [CrossRef] [PubMed] [Google Scholar]
- Multicentre randomized study on the effect of a low-protein diet on the progression of renal failure in childhood: One-year results. European study group for nutritional treatment of chronic renal failure in childhood. Miner Electrolyte Metab. 1992;18:303-8.
- [PubMed] [Google Scholar]
- Protein restriction for children with chronic renal failure. Cochrane Database Syst Rev 2007:CD006863. doi: 10.1002/14651858.CD006863
- [CrossRef] [PubMed] [Google Scholar]
- Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int. 2012;32:S32-86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The dietary approach to the treatment of the rare genetic tubulopathies gitelman’s and bartter’s syndromes. Nutrients. 2021;13:2960.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Growth hormone and insulin-like growth factor dysregulation in pediatric chronic kidney disease. Horm Res Paediatr. 2021;94:105-14.
- [CrossRef] [PubMed] [Google Scholar]
- Growth hormone for children with chronic kidney disease. In: Cochrane database of systematic reviews. John Wiley & Sons, Ltd; 2006.
- [Google Scholar]
- Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease. Nat Rev Nephrol. 2019;15:577-89.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of growth hormone treatment on the adult height of children with chronic renal failure. German study group for growth hormone treatment in chronic renal failure. N Engl J Med. 2000;343:923-30.
- [CrossRef] [PubMed] [Google Scholar]
- Hypothyroidism in children with chronic kidney disease attending a tertiary care center. Saudi J Kidney Dis Transpl. 2021;32:1722-6.
- [CrossRef] [PubMed] [Google Scholar]
- Kidney disease and thyroid dysfunction: The chicken or egg problem. Pediatric Nephrology. 2022;37:3031-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Puberty and chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:372-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pubertal development in children with chronic kidney disease. Pediatric Nephrology. 2017;32:949-64.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing bone mineralization in children with chronic kidney disease: What clinical and research tools are available? Pediatric Nephrology. 2020;35:937-57.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bone evaluation in paediatric chronic kidney disease: Clinical practice points from the european society for paediatric nephrology CKD-MBD and Dialysis working groups and CKD-MBD working group of the ERA-EDTA. Nephrol Dial Transplant. 2021;36:413-25.
- [CrossRef] [PubMed] [Google Scholar]
- Anemia in chronic kidney disease. Pediatric Nephrology. 2018;33:227-38.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical practice guidelines for anemia in chronic kidney disease: Problems and solutions. A position statement from kidney disease: Improving global outcomes (KDIGO) Kidney Int. 2008;74:1237-40.
- [CrossRef] [PubMed] [Google Scholar]
- Anemia in children with chronic kidney disease. Nat Rev Nephrol. 2011;7:635-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The brain in pediatric chronic kidney disease–the intersection of cognition, neuroimaging, and clinical biomarkers. Pediatric Nephrology. 2020;35:2221-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neurocognition in pediatric chronic kidney disease: A review of data from the chronic kidney disease in children (CKiD) study. Semin Nephrol. 2021;41:446-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- One-year mortality rates in US children with end-stage renal disease. Am J Nephrol. 2015;41:121-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mortality and causes of death of end-stage renal disease in children: A dutch cohort study. Kidney Int. 2002;61:621-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol. 2012;23:578-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- US Renal Data System: USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2011.
- Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141:191-7.
- [CrossRef] [PubMed] [Google Scholar]
- Vaccine administration in children with chronic kidney disease. Vaccine. 2014;32:6601-06.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease in children and adolescents. Pediatr Rev. 2014;35:16-29.
- [CrossRef] [PubMed] [Google Scholar]
- Immunizations in children with chronic kidney disease. Pediatric Nephrology. 2012;27:1257-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Government of Western Australia Department of Health, Renal Health Network, 2017.
- Red blood cell transfusions and the risk of allosensitization in patients awaiting primary kidney transplantation. Transplantation. 2014;97:525-33.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for Living Donor Transplatation. British Transplantation Society 2018.
- Paediatric kidney transplantation: Towards a framework for pretransplant urological evaluation. Pediatr Transplant. 2022;26:e14299.
- [CrossRef] [PubMed] [Google Scholar]
- Prescribing peritoneal dialysis for high-quality care in children. Perit Dial Int. 2020;40:333-40.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int. 2012;32:S32-86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- End-stage kidney disease in infancy: An educational review. Pediatric Nephrology. 2020;35:229-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Transition of kidney care at 18: Challenges and practical solutions for India. Indian J Nephrol. 2023;33:325-32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical profile and outcome of emergencies in pediatric chronic kidney disease. Indian Pediatr. 2022;59:31-4.
- [PubMed] [Google Scholar]
- Paediatric nephrology in under-resourced areas. Pediatric Nephrology. 2022;37:959-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors and rate of progression of CKD in children. Kidney Int Rep. 2019;4:1472-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Challenges of access to kidney care for children in low-resource settings. Nat Rev Nephrol. 2021;17:33-45.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: A systematic review. Lancet Glob Health. 2017;5:e408-17.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990-2010. JAMA. 2013;309:1921-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








