Osseous Metaplasia in Renal Allograft
Corresponding author: Anila A. Kurien, Renopath, Center for Renal and Urological Pathology, No 27 and 28, VMT Nagar, Kolathur, Chennai, Tamil Nadu, India. E-mail: anila_abraham08@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Malathi CV, Venkatesh Rajkumar S, Jansi Prema KS, Kurien AA. Osseous Metaplasia in Renal Allograft. Indian J Nephrol. 2024;34:537-8. doi: 10.25259/ijn_527_23
Osseous metaplasia (OM) is the presence of heterotopic bone tissue in the extraskeletal soft tissue. OM was described in many nontransplant organs like parotid, skin, lung, labyrinth, endometrium, and kidney.1 OM is a rare finding in the renal allograft biopsy.
A 35-year-old male developed end-stage renal disease as a result of chronic interstitial nephritis. The patient underwent live donor renal transplantation and was on triple drug regimen of tacrolimus, mycophenolate mofetil, and steroids. Three years posttransplant, the patient developed acute pyelonephritis, which was treated with appropriate antibiotics, following which the graft function stabilized. Five months later, he again developed graft dysfunction, with serum creatinine of 2 mg/dl. Serum calcium was 9.3 mg/dl. The kidney was normal on ultrasonogram; no calcification was seen. A renal biopsy was performed. Light microscopy showed eleven glomeruli, 2 were globally sclerotic. All the viable glomeruli were of normal cellularity. The capillary loops were patent and no fibrin thrombus was noted in the lumen. Glomerular basement membrane appeared normal. Interstitial fibrosis involved 50% of the sampled cortex. Lymphocytic infiltration was seen in the fibrosed cortex. There was a focus in the renal cortex where woven bone formation was noticed [Figure 1]. The osteocytes located in the lacunae were appreciated under higher magnification. Focal mineralization was also observed [Figure 2]. There were no hematopoietic cells or adipocytes in the region of bone formation. The biopsy showed no features of rejection, including tubulitis, peritubular capillaritis, or vasculitis. C4d immunostaining was negative. On follow-up, his graft function is stable, with serum creatinine maintained at 2 mg/dl.
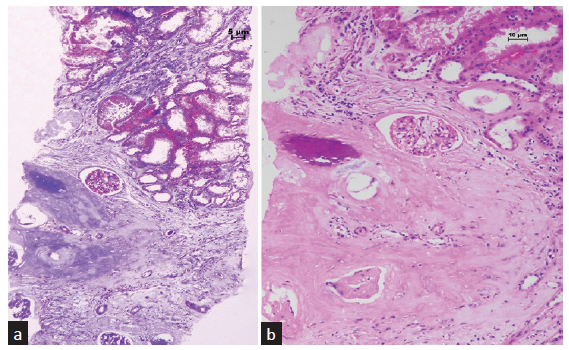
- (a) Woven bone is seen in the renal parenchyma. The surrounding tissue shows fibrosis of the interstitium (Masson trichrome stain, 100×). (b) There is a focus of mineralization within the woven bone (hematoxylin and eosin stain, 200×).
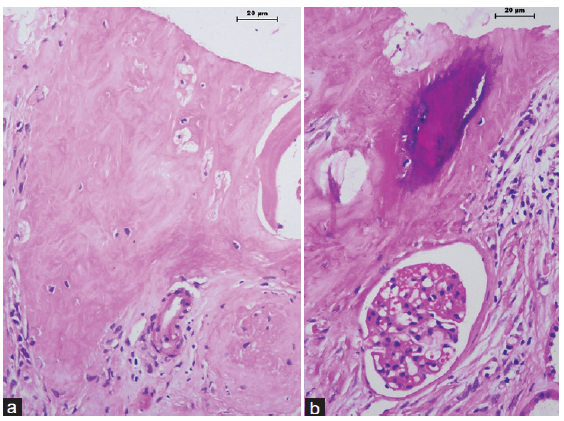
- There are (a) osteocytes within lacunae and (b) a focus of mineralization (hematoxylin and eosin stain, 400×).
OM results from the differentiation of mesenchymal pluripotent cells into bone tissue. The heterotopic bone matrix can be mineralized and be associated with adipocytes and hematopoietic cells. The osseous differentiation, considered aberrant tissue repair, requires a suitable environment and osteogenic signals. Various experimental studies have described the occurrence of OM in association with ischemia and inflammation. OM can be associated with the presence of hematopoietic elements (myeloid metaplasia) as reported by Azhir et al.2
There are limited cases of OM reported in the renal allograft [Table 1]. Majority of them were detected following allograft nephrectomy.1-4 The time taken for the appearance of OM varied between 6 months and 5 years. OM was observed during autopsy in one case.5 All except our case had concomitant features of rejection. Chronic inflammation or ischemic changes were present in all the cases.6 OM in our patient is likely to be the sequela of pyelonephritis. Similar to our patient, Bataille et al.1 observed OM in association with recurrent pyelonephritis.1 They hypothesized that OM resulted from chronic ischemia or inflammation following infection or as an immunologic process.
| Author | Age at Tx | Specimen type | Native kidney disease | Rejection | Type of donor | Location of osseous metaplasia |
|---|---|---|---|---|---|---|
| Current case | 35 | Allograft biopsy | Chronic interstitial nephritis | No rejection | Live | Renal cortex |
| Bataille et al.1 | 21 | Graft nephrectomy | Interstitial nephritis | Chronic | Deceased | Renal cortex |
| Azhir et al.2 | 28 | Graft nephrectomy | Diabetic Nephropathy | Acute on chronic | Live | Renal cortex and medulla |
| Tousignant et al.3 | 15 | Graft nephrectomy | Renal dysplasia | Acute on chronic | Deceased | Renal cortex |
| Chan et al.4 | 43 | Graft nephrectomy | IgA Nephropathy | Chronic | Deceased | Renal parenchyma |
| Makhoba et al.5 | 11 | Autopsy | Unknown | Chronic | NA | Renal parenchyma |
| Sanders et al.6 | 22 | Allograft biopsy | FSGS Collapsing variant | Acute on chronic | Deceased | Renal cortex |
The calcification detected in the allograft by imaging studies could be due to nephrolithiasis, ectopic calcification, or OM.1 Nephrolithiasis is located in renal pelvis. Ectopic calcification is the inappropriate biomineralization in soft tissues with deposition of calcium salts and can occur in uremic patients due to mineral imbalance. OM is different from ectopic calcification. Unlike OM, bone formation, osteocytes, or hematopoietic elements are absent in ectopic calcification.
OM in the renal allograft is rare. Though not directly implicated in the graft dysfunction, its presence in the biopsy hints toward chronic injurious stimuli like inflammation, ischemia, or infection in the allograft.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Osseous metaplasia in a kidney allograft. Nephrol Dial Transplant. 2010;25:3796-8.
- [CrossRef] [PubMed] [Google Scholar]
- Osseous and myeloid metaplasia in a failed renal allograft. Kidney Int. 2007;71:829-30.
- [CrossRef] [PubMed] [Google Scholar]
- Bone metaplasia associated with chronic allograft nephropathy. Kidney Int. 2006;70:407.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic appearance of renal transplant osseous metaplasia: Case report. Can Assoc Radiol J. 1999;50:390-2.
- [PubMed] [Google Scholar]
- Osseus metaplasia in chronic renal allograft rejection. Afr J Nephrol. 2017;20:45-7.
- [CrossRef] [Google Scholar]
- Osseus metaplasia in kidney allografts as a paradigm of regenerative medicine principles. Exp Clin Transplant. 2014;12:371-3.
- [CrossRef] [PubMed] [Google Scholar]






