Translate this page into:
Renal Allograft Cortical Necrosis in a COVID-19 Positive Patient
Address for correspondence: Dr. Karthikeyan Balasubramanian, Department of Nephrology, Saveetha Medical College and Hospital, Thandalam, Chennai, Kancheepuram - 602105, Tamil Nadu, India. E-mail: karthiprakash1986@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The incidence of acute kidney injury (AKI) has been reported to be higher in kidney transplant recipients infected with SARS-CoV-2 compared with the general population. Here, we report a case of cortical necrosis in the graft kidney due to COVID infection in a patient with stable graft function over the years. The patient was started on hemodialysis and treated with steroids, and anticoagulants for COVID infection. Later, he had gradual improvement in his graft function and became dialysis independent on follow up.
Keywords
Cortical necrosis
COVID
renal allograft
Introduction
The incidence of acute kidney injury (AKI) has been reported to be higher in kidney transplant recipients infected with SARS-CoV-2 compared with the general population.[1] A European study conducted by the ERA-EDTA from the French and Spanish registries yielded an average infection rate of 14/1000 transplants at risk.[2–4] Advanced age, male gender, severity of respiratory impairment, requirement for mechanical ventilation, pre-existing chronic renal failure, co-infection with other organisms, and systemic inflammatory response have been described as the main risk factors associated with AKI.[5,6]
Histologically, the most common finding on renal biopsy of non-renal transplant patients infected with SARS-CoV-2 is acute tubular necrosis (ATN).[7] Collapsing glomerulopathy,[8] thrombotic microangiopathy (TMA), minimal change disease,[9] and pauci-immune crescentic glomerulonephritis[10] have also been variably described. In addition to the above findings,[11] in renal transplant recipients, cortical necrosis,[12] and a few cases of T cell or B cell mediated rejection have been described.[13] Here we are reporting a case of cortical necrosis in graft kidney.
Case Presentation
A 47-year-old male renal allograft recipient with a history of hypertension was admitted to our hospital with c/o cough, fever, and breathlessness for five days. On evaluation, he was found to have COVID-19 infection detected by RT-PCR. The patient’s native kidney disease was chronic interstitial nephritis due to vesico-ureteric reflux and he had received an ABO compatible and well-matched kidney from a living donor 17 years ago. Prior to transplantation, he was on a continuous ambulatory peritoneal dialysis (CAPD) regimen for six months. For the past 17 years, he had excellent graft function and no episodes of graft dysfunction. His serum creatinine done two weeks prior to the day of admission was 0.9 and at the time of presentation, he was on triple immunosuppression regimen consisting of tab. prednisolone 5 mg once a day, tab. cyclosporine total 75 mg per day, and tab. azathioprine 100 mg daily.
At the time of admission, the patient had a chest CT severity score of 22 [Figure 1a and b]. While he usually had urine output of 1.5 to 2 liters per day, at the time of admission, urine output dropped to about 500–600 ml per day. He was admitted to the ICU and lab investigations revealed that his serum creatinine was 2.7. Till the day he was admitted, the patient had been taking his routine immunosuppressive drugs as per schedule. Over the next two days, his s. creatinine had increased to 5.6, D-dimer was 2,300 ng/ml, CRP was 42 mg/L, LDH was 512 U/L, and his output dropped to 70–100 ml per day. There was no hypotension during the hospital stay. His peripheral smear showed leukocytosis and mild thrombocytopenia. With possible diagnosis of either ATN or acute rejection, patient was initiated on hemodialysis through a right internal jugular vein dialysis catheter. His azathioprine was stopped. As part of COVID-19 management, he received inj. methylprednisolone 125 mg once a day, inj. remedesvir 200 mg on day 1 and 100 mg from day 2 to day 5, and inj. heparin 5000 U thrice day. After 5 sessions of hemodialysis and one day of inj. heparin being on hold, patient underwent an ultrasonography (USG)-guided renal biopsy of the graft.
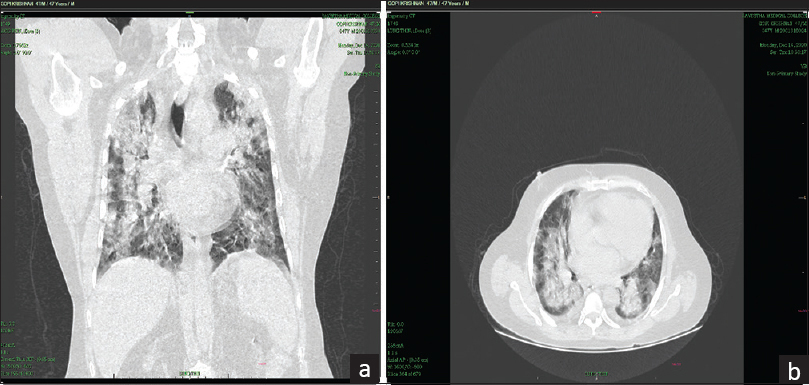
- (a and b) HRCT thorax showing severe lung involvement of COVID pneumonia (severity score: 22/25)
Histology
The graft biopsy sample on light microscopy showed glomeruli and tubules with coagulative necrosis, extensive neutrophilic infiltration, and nuclear debris, with 95% of biopsied sample showing evidence of cortical necrosis with a medium-sized vessel showing an organized thrombus [Figures 2 and 3]. Immunofluorescence was negative for immunoglobulins, C3, and C4d.

- Graft renal cortical tissue showing necrosis

- Interlobular artery showing thrombosis
Follow up
After 10 days of ICU stay, patient was shifted to the ward and discharged with s. creatinine at 5.2, anticoagulation with apixaban 2.5 mg OD, and him requiring twice weekly hemodialysis. At the time of discharge, he had a urine output of 120–150 ml per day. Over the next one month, his urine output gradually started to improve and by one month after discharge, he had urine output of 1,200 ml per day. His hemodialysis was stopped and his IJV dialysis catheter was removed and patient was being followed up on OPD basis. Currently, 6 months since his discharge, patient has had stable s. creatinine of 3.1 with a urine output of 2,000 ml per day.
Discussion
It has been established that the novel COVID-19 virus is associated with a severely prothrombotic state[14] and in particular, ICU patients have a 10%–30% higher incidence of venous thromboembolism than patients admitted in general wards.[15] The pathophysiology behind the prothrombotic state is incompletely understood. The proposed mechanism is activation of endothelial cells directly by the virus or the resultant cytokine storm leading to endothelial damage, platelet activation and hyperactivation of the coagulation cascade.[16] The most commonly reported thrombotic complication is pulmonary embolism (PE)[17] and with the incidence of deep vein thrombosis (DVT) being far less than PE in these patients, it has been proposed that the thrombotic events are not emboli but rather primary thromboses.[18]
Renal cortical necrosis (RCN) causes irreversible kidney damage and may present as oliguric/anuric renal failure, hematuria, flank pain, and most cases progress to ESRD.[19] Histologically, RCN is classified into diffuse and patchy, and patients with diffuse RCN end up being dialysis-dependent or develop ESRD earlier[20,21] than patients with patchy RCN, who are characterized by a better renal recovery.[21] In the case of our patient, prothrombotic state associated with COVID-19, in spite of him being on prophylactic anticoagulation, has probably caused RCN. Considering the diffuse nature of the RCN, his chances of complete renal recovery ought to be lower. However, the patient regained renal function and was dialysis independent by the end of one month.
Data about biopsy findings in renal transplant recipients with graft dysfunction is limited to a series of case reports and the commonest findings were ATN, acute rejection and TMA. Cortical necrosis was reported in isolated case reports.[9] Considering the increased prothrombotic state of COVID-19 especially in the ICU setting, and the not so common practice of performing renal biopsy in COVID positive patients with AKI, and renal transplant patients due to their immunosuppressed state being more prone for viral induced endothelial injury, perhaps RCN is far more common than reported and should be considered as a possible etiology for acute graft dysfunction.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Kidney transplantation in the times of COVID-19-A literature review. Ann Transplant. 2020;25:e925755.
- [Google Scholar]
- Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540-8.
- [Google Scholar]
- COVID19 in transplant recipients:The Spanish experience. Am J Transplant. 2021;21:1825-37.
- [Google Scholar]
- An initial report from the French SOT COVID registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549-58.
- [Google Scholar]
- Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2020;77:204-15. e1
- [Google Scholar]
- Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209-18.
- [Google Scholar]
- Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219-27.
- [Google Scholar]
- Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease:A report of 2 cases. BMC Nephrol. 2020;21:326.
- [Google Scholar]
- Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis. 2020;77:82-93. e1
- [Google Scholar]
- COVID-19–associated kidney injury:A case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31:1948-58.
- [Google Scholar]
- Collapsing glomerulopathy affecting native and transplant kidneys in individuals with COVID-19. Nephron. 2020;144:589-94.
- [Google Scholar]
- Renal infarct in a COVID-19- positive kidney-pancreas transplant recipient. Am J Transplant. 2020;20:3221-4.
- [Google Scholar]
- Unusually high rates of acute rejection during the COVID-19 pandemic:Cause for concern? Kidney Int. 2020;98:513-4.
- [Google Scholar]
- Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40:2033-44.
- [Google Scholar]
- Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14.
- [Google Scholar]
- Thrombosis and coagulopathy in COVID-19:An illustrated review. Res Pract Thromb Haemost. 2020;4:744-51.
- [Google Scholar]
- The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore). 2019;98:e15833.
- [Google Scholar]
- Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-7.
- [Google Scholar]
- Diagnostic procedures and long term progress in bilateral cortical necrosis. Kidney Int. 1973;4:390-400.
- [Google Scholar]
- Prolonged oliguria with survival in acute bilateral cortical necrosis. BMJ. 1968;4:220-2.
- [Google Scholar]







