Translate this page into:
Plasmodium vivax Malaria Presenting as TMA and Acute Cortical Necrosis: A Case Series
-
Received: ,
Accepted: ,
How to cite this article: Chakrabarti U, Chaturvedy M, Sabari B, Jhorawat R, Nalwa A, Bajpayee A, et al. Plasmodium vivax Malaria Presenting as TMA and Acute Cortical Necrosis: A Case Series. Indian J Nephrol. 2024;34:165–8. doi: 10.4103/ijn.ijn_206_23
Dear Editor,
Acute kidney injury (AKI), though commonly reported with falciparum malaria, is not uncommon with vivax. The association among renal failure, anemia, thrombocytopenia, and jaundice is a common finding in severe malaria and can mimic thrombotic microangiopathy (TMA).1
We present three patients with Plasmodium vivax malaria who presented with TMA.
Case 1- Patient A, a 25-year-old female, presented with fever 10 days back lasting for 8 days, loose stools which persisted for 5 days, and anuria and orthopnea since last 1 day. She was evaluated outside and her baseline parameters are enumerated in Table 1. She was found to be positive for P. vivax on rapid card test and received artesunate, followed by oral artemether-lumefantrine combination and primaquine. In view of her decrement in hemoglobin and platelet counts and nonrecovery of renal function, TMA was suspected. She underwent one session of plasmapheresis, following which her platelet counts normalized. Kidney biopsy was done in view of persistent renal dysfunction and dialysis dependence for 2 weeks. It revealed focal acute cortical necrosis with schizonts of P. vivax in thrombosed glomeruli with TMA [Figure 1a], myoglobin casts, acute tubular injury in the non-necrotic tubules with an interstitial fibrosis and tubular atrophy (IFTA) of 15%. She was continued on maintenance hemodialysis, with the urine output being 200 ml at discharge. She was continued on dialysis for 3 weeks since her initial presentation, following which there has been normalization of her urine output and renal parameters.
| Parameters | Patient A | Patient B | Patient C |
|---|---|---|---|
| Age/sex | 25/F | 35/F | 22/F |
| Admission after onset of fever | 10 days | 20 days | 10 days |
| Hb (g/dl) | 7.5 | 5.7 | 3.9 |
| TLC (/μl) | 9570 | 7700 | 4300 |
| Platelets (/μl) | 39,000 | 43,000 | 29,000 |
| Schistocytes | 2%-3% | 0.9% | <1% |
| U/Cr (mg/dl) | 266/8 | 68/1.62 | 116/5.1 |
| T bil/D bil (mg/dl) | 1.31/0.64 | 0.8/0.2 | 3.17/2 |
| AST/ALT/ALP (lU/l) | 50/52/122 | 20/43/180 | 551/292/162 |
| LDH (IU/l) | 2231 | No baseline 453 (inhouse) | 3196 |
| T pro/ALB (g/dl) | 7.65/4.43 | 6/3.15 | 6.35/2.5 |
| INR | 1.2 | 1.3 | 1.2 |
| Urine microscopy | Pro3+, 250 RBC | Pro-, RBC- | Anuric |
| G6PD deficiency | - | - | - |
| U. O. at presentation | Anuric | 800 ml | Anuric |
| Neurological manifestation | - | 2 episodes of GTCS (MRI- normal) | - |
| Hypertension | No | No | No |
| Transfusions | 1 PC | 4 PC, 3 FFP | 4 PC, 4 RDP |
| IV artesunate | Yes | Yes | Yes |
| Oral primaquine | Yes | Yes | Yes |
| Plasmapheresis | 1 session | - | 1 session |
| HD sessions before kidney biopsy | 5 | 7 | 6 |
| Biopsy finding | Focal acute cortical necrosis, TMA, myoglobin casts, ATI | Patchy acute cortical necrosis, TMA, ATIN | Diffuse cortical necrosis, TMA, ATI |
| IFTA | 15% | 40% | Infarcted tubules |
| Urine output at discharge | 200 ml | 1200 ml | Nil |
| HD vintage (from presentation) | 3 weeks | 2 weeks | Ongoing |
| Follow-up after 3 months | |||
| Present status | Off HD | Off HD | On MHD |
| Hb (g/dl) | 12.9 | 9.9 | 9 |
| U/Cr (mg/dl) | 41/1.2 | 93/4.14 | 74/5.23 |
| Urine microscopy | PRO-, RBC- | PRO3+ | PRO1+, 1-2 RBC |
ALB=albumin, ALP=alkaline phosphatase, ALT=alanine transaminase, AST=aspartate transaminase, ATI=acute tubular injury, ATIN=acute tubulointerstitial nephritis, FFP=fresh frozen plasma, G6PD=glucose-6-phosphate dehydrogenase, GTCS=generalized tonic clonic seizure, Hb=hemoglobin, HD=hemodialysis, IFTA=interstitial fibrosis, tubular atrophy, INR=international normalized ratio, IV=intravenous, LDH=lactate dehydrogenase, MHD=maintenance hemodialysis, PC=packed cell, RBCs=red blood cells, RDP=random donor platelet, T bil/D bil=total bilirubin, direct bilirubin, TLC=total leukocyte count, TMA=thrombotic microangiopathy, T pro=total protein, U/Cr=urea/creatinine, U. O. = urine output
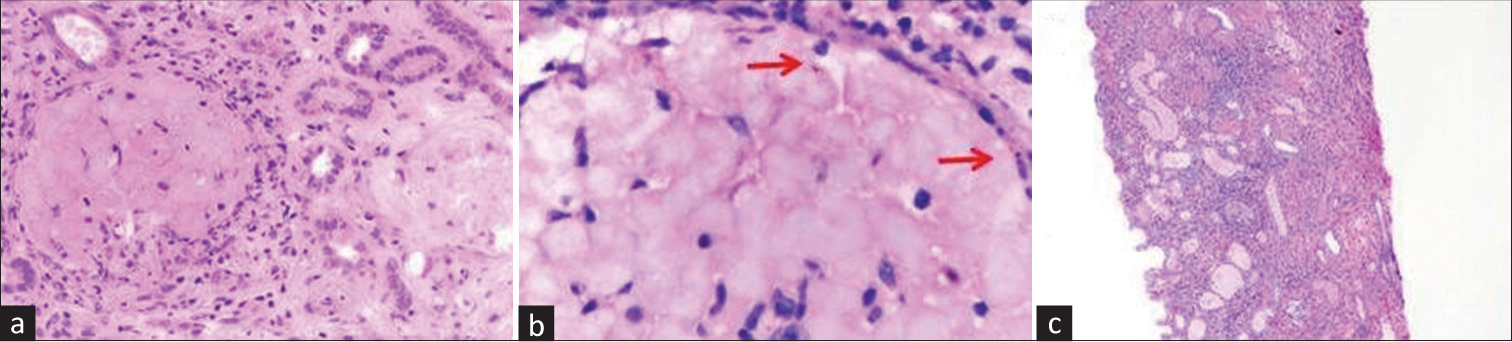
- (a) Glomeruli are infarcted and bloodless (H and E, ×400). (b) Presence of malarial schizonts (red arrows) in necrosed glomeruli (H and E, oil immersion.) (c) Low-power view showing cortical necrosis of the tubules and interstitium (H and E, ×100). H and E = hematoxylin and eosin.
Case 2- Patient B, a 35-year-old female, presented with history of fever 20 days back associated with headache and vomiting, which lasted for 7 days, along with diminished urine output. She was admitted outside, where she was found to be P. vivax positive on rapid card test and treated with intravenous (IV) artesunate along with four units of packed cell and three units of fresh frozen plasma (FFP) transfusion, and she underwent six sessions of hemodialysis. She also gave history of two episodes of generalized convulsions 1 day before presentation to our hospital. Though there was an improvement of urine output, renal parameters did not improve at 2 weeks. She was subjected to kidney biopsy, which revealed patchy acute cortical necrosis with schizonts in thrombosed glomeruli [Figure 1b], TMA, and moderate to severe acute tubulointerstitial nephritis with an IFTA of 40%. She had been off hemodialysis on discharge with an adequate urine output. Follow-up after 3 months showed the patient had partial recovery of her renal parameters.
Case 3- Patient C, a 22-year-old female, presented with history of fever 10 days back which persisted for 2–3 days, vomiting and loose stools, diminished urine output since 7 days, and generalized body swelling since 2 days. She was evaluated outside and found to be positive for P. vivax on rapid card test. She received two units of packed cell and four units of platelet transfusion and underwent two sessions of hemodialysis before presenting to us. She was normotensive and anuric on presentation and was continued on IV artesunate and primaquine and maintenance hemodialysis. She also received two units of packed cell transfusion. She was given a session of plasma exchange, following which there was an improvement of hematological parameters and treatment was discontinued after a solitary session. However, she continued to be anuric, for which kidney biopsy was done with duration of renal dysfunction and dialysis dependence of 2 weeks. Biopsy revealed diffuse acute cortical [Figure 1c] with schizonts of P. vivax in thrombosed glomeruli, TMA, and acute tubular injury in the non-necrotic tubules. She continued to be anuric and was discharged with the advice of maintenance hemodialysis.
We had three female patients who had documented Plasmodium vivax infection detected in rapid card test with biopsy showing evidence of TMA with varying degree of cortical necrosis. The pathophysiology of TMA in vivax malaria can be explained by the following:
Parasitized red blood cells adhering to the adjacent healthy erythrocytes, platelets, and the capillary endothelium (cytoadherence) which results in the formation of intravascular clusters and rosettes. The infected RBCs can sequester in the deep vascular beds of the kidney disrupting microcirculation as well as peripherally pool, leading to anemia, thrombocytopenia, tissue hypoxia.2
Change in erythrocyte magnesium-activated ATPase following vivax infection leading to a decrease in intracellular sodium concentration which in turn would lead to calcium influx altering the red cell deformability, shortening red cell lifespan contributing to peripheral pooling and organ sequestration causing kidney injury and TMA. The cell free hemoglobin resulting from hemolysis of both healthy and infected RBCs mediates red cell membrane phospholipid peroxidation which not only changes its deformability but also generates prostaglandin isomers which act as potent renal vasoconstrictors reducing renal blood flow and renal function.3
Similar to shiga toxin the malarial toxin can alter endothelial cell integrity leading to microvasculature disruption and TMA. The inflammatory response and cytokine production with P.vivax infection has been found to be greater than that seen in P. falciparum infections with a similar parasite biomass.4 This explains the association of TMA particularly with vivax in comparison to falciparum although kidney involvement is assumed to be more severe with the latter.
P. vivax infection is found to be associated with elevated thrombomodulin, Von Willebrand factor (VWF) and procoagulant activity which in turn results in intravascular coagulation and endothelial inflammation through increased formation of ultra-large VWF and platelet aggregates.5
A very typical finding in all our three cases has been the presence of malarial schizonts in the thrombosed glomeruli which is not that commonly encountered across the reported cases of malarial TMA. The next question that arises is why only few patients with vivax malaria would develop TMA and why most of the cases have been reported majorly from India. Whether it’s the ethnic susceptibility or a genetic predisposition to TMA remains unanswered. We didn’t work up our patients in terms of any genetic analysis or complement mutations which could have thrown some light in the matter.
Cortical necrosis in malaria characterises a more severe kidney injury and is generally associated with non- recovery of renal function and consequent development of end-stage kidney disease. Factors contributing include hypovolemia, vasoconstriction, hemolysis, erythrocyte parasitemia, immune complexes deposition in glomeruli, microcirculation dysfunction (due to cytoadherence of parasite erythrocytes) and rhabdomyolysis.6 In our case series the outcome correlated with the degree of cortical necrosis. The one having focal cortical necrosis had complete recovery of renal function,one with patchy cortical necrosis landed up in CKD5ND while the other having diffuse cortical necrosis developed ESRD.
Poor prognostic factors associated with malarial AKI includes a delayed referral, high parasite load, multi-system involvement, younger age, severe jaundice, hypotension, severe anemia, and oliguria.3 The same factors accounted for poor prognosis in our series as well where in the the patient C who landed up in ESRD was the youngest of the three, had kidney and liver involvement,severe anemia and was anuric on presentation.
Assounga et al. found up to 12% of patients developed chronic impairment in kidney function after malarial AKI.7 Renal insufficiency in severe malarial infection commonly develops 3–7 days after the onset of fever; serum creatinine typically improves in 17 ± 6 days. Around 80% of patients can develop non-oliguric AKI whereas in those with oliguric AKI, the oliguria can persist for weeks.8
Plasma exchange can be tried in patients with TMA in vivax malaria where there can be renal recovery,9 however the results are not uniform. In the case series by Bhadauria et al.,9 four patients of vivax with TMA were subjected to plasmapheresis ranging from 5-8 sessions showing improvement in renal functions and dialysis independency. The end point of discontinuation of plasmapheresis needs to be defined in this setting as in whether it’s the hematological recovery that is to be followed or is it the renal recovery which should be taken into account. In our case series two patients (A and C) were given a single session of plasmapheresis. Although there was no renal recovery, with a single session the hematological parameters improved following which it was discontinued. Though one patient had complete recovery of renal function (A), the other (C) landed up in ESRD.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Thrombotic microangiopathy and acute kidney injury following vivax malaria. Clin Exp Nephrol. 2013;17:66-72.
- [CrossRef] [PubMed] [Google Scholar]
- Sequestration and its discontents: Infected erythrocyte-endothelial cell interactions in Plasmodium falciparum malaria. Res Immunol. 1993;144:740-5.
- [CrossRef] [PubMed] [Google Scholar]
- Malarial acute renal failure. J Am Soc Nephrol. 2000;11:2147-5.
- [CrossRef] [PubMed] [Google Scholar]
- Lung injury in vivax malaria: Pathophysiological evidence for pulmonary vascular sequestration and post treatment alveolar-capillary inflammation. J Infect Dis. 2007;195:589-96.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of thrombomodulin, intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin in the acute phase of Plasmodium vivax malaria. Am J Trop Med Hyg. 1999;60:248-50.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmodium vivax causing acute kidney injury: A foe less addressed. Pak J Med Sci. 2015;31:1472-5.
- [CrossRef] [PubMed] [Google Scholar]
- Etiology and outcome of acute renal failure in children in Congo-Brazzaville. Saudi J Kidney Dis Transpl. 2000;11:40-3.
- [Google Scholar]
- Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr Opin Infect Dis. 2018;31:69-77.
- [CrossRef] [PubMed] [Google Scholar]
- P. vivax malaria presenting as thrombotic microangiopathy. J Assoc Physicians India. 2017;65:28-31.
- [Google Scholar]






