Translate this page into:
Outcomes of Spousal versus Parental Donor Kidney Transplants: A Comparative Study
Address for correspondence: Dr. Benil Hafeeq, Department of Nephrology, Iqraa International Hospital and Research Centre, Kozhikode - 673 009, Kerala, India. E-mail: benilhafeeq@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Parents and spouse constitute 70% of organ donors in India. Some centres use induction immunosuppression (IS) for all spousal transplants considering it as an immunologically high risk. This study was designed to compare the outcomes of transplant recipients who received parental donors (PDs) and spousal donors (SDs) without any induction IS.
Methods:
It was a retrospective study conducted at a tertiary care hospital in South India. Adults aged 18 years or above who underwent renal transplantation from a SD or PD between January 2006 and December 2016 were included in the study.
Results:
Our study included 154 patients with PDs and 75 patients with SDs. The mean recipient age of the PD group was 27.79 ± 6.85 years and of the SD group was 45.62 ± 7.96 years (P < 0.001). However, the follow-up period was significantly higher for the PD group (P < 0.05). There was no significant difference between acute rejection, patient loss, mean survival, graft survival (uncensored), and death censored graft survival between two groups.
Conclusion:
The outcomes of immunologically low-risk transplant recipients who have received PD and SD are similar and induction immunosuppression can be avoided in these patients.
Keywords
Kidney transplant
outcomes
parental donor
spousal donor
Introduction
Living donors constitute more than 90% of renal transplantation in India. Albeit the deceased donor program is increasing due to a myriad of government initiatives, projected trend shows that living donation is going to be the mainstay for a longtime in the Indian transplant scenario.[1] Apart from the availability of donors, the other two tantamount hurdles of transplantation in the sub-continent are the risk of infections and financial affordability.[23] In India, only after the implementation of the Human Organ Transplant Act in 1995 the spouses started to donate in increasing numbers.[4] Parents constitute more than 50% of living donors, and spouses account for 20%-30% of donors in India, akin to many other Asian countries.[56] Studies have shown that spousal donation has outcomes comparable to living related donor (RD).[7] Spousal donors (SDs) usually facilitate more age-matched donors compared to parental donors (PDs). Many transplant centers consider the SD transplantation as immunologically high risk due to increased HLA mismatches and subject recipients to more intense immunosuppression (IS) with perioperative induction using Thymoglobulin or IL2 receptor blockers that makes them vulnerable to opportunistic infections. The role of induction IS in immunologically low-risk living donor candidates in the current Tac-MMF-based era is still not robust.[8] Most of the studies that have compared the outcome of spousal donors with living RDs had been from the era prior to current IS and histocompatibility testing methods, of short-term follow-ups and have used heterogeneous IS protocols.[91011] The IS protocol at our center had been the same for all immunologically low-risk individuals from the anti-HLA antibody standpoint documented by a negative anti-HLA antibody Class I and Class II screen by Flow panel reactive antibody (PRA) beads or multiple antigen screen beads on Luminex platform. The immunologically low-risk patients with negative antibody screen did not receive any induction IS irrespective of the donor relation. In our current study, we have compared the outcomes of immunologically low-risk transplant recipients who have received PDs and SDs without any induction IS. Presumed risk of increased immune injury and inferior outcome in SDs could be teased out only if we use the same IS protocol and have long-term follow up. We have analyzed the incidence of rejection, opportunistic infections, graft loss and patient loss in both groups.
Methods
It was a retrospective study conducted at a tertiary care hospital in South India. Adults aged 18 years or above who underwent renal transplantation from a SD or PD between January 2006 and December 2016 were included in the study. The data were collected during patient visits in outpatient clinics. Patients undergoing ABO-incompatible transplantation, patients received induction therapy, and female recipients with husband donors who had pregnancy or abortion as sensitizing event were excluded from the study.
Pre-transplant evaluation and post-transplant management protocol
The lower limit of donor age in our program is 21 years. Potential donors with impaired fasting glucose levels or hypertension needing more than two antihypertensive medications are declined. We do not follow 'Age-adapted GFR', and eGFR of 60 mL/mt/1.73 m 2 (according to CKD-EPI equation) is defined as the acceptable lower limit for kidney donation. All renal donors also underwent a Tc99m DTPA radionuclide scan for estimation of split and total GFR using the Gate's method that tends to slightly overestimate GFR compared to other methods.
All patients underwent immunological profiling to be identified as “Low Immunological Risk” (LIR) that varied through three phases of histocompatibility testing. From 2006 to September 2012 patients were identified as LIR if they did not have any sensitizing event and had negative Complement-dependent cytotoxic (CDC) crossmatch and AMS-ELISA-crossmatch, which used donor lysate on and ELISA platform. From September 2012 to August 2013, all recipients underwent anti-HLA antibody screen by Luminex platform along with CDC crossmatch, both to be negative and identified as LIR. From August 2013, all recipients underwent immunological profiling on all three platforms: Cytotoxic crossmatch, Flow crossmatch and antibody screen using mixed antigen bead analysis by Luminex platform; all the three are negative to be called LIR. All patients with LIR were not given induction IS. None of the recipients underwent post-transplant monitoring for the anti-HLA antibody.
Immunosuppression protocol
None of the patients with LIR received any induction IS as per protocol. The patients who received thymoglobulin as induction due to delayed graft function (DGF) had been excluded from the study. Recipients were started on triple IS: Prednisolone 10 mg, Tacrolimus 0.04 mg/kg body wt. and MMF 500 mg BID from the day 'minus 4% of date of transplantation. All received Injection Methyl prednisolone from the day 'minus 1' to fourth post-operative day. After transplantation, all patients were maintained on triple IS. Tacrolimus level was kept around 7-10 ng/dL for the first 1 month and subsequently around 4-7 ng/dL. Prednisolone was tapered from 30 mg on second post-operative week to 5 mg by 10th post-operative week. All patients were maintained on MMF 1 g per day or Azathioprine 2-2.5 mg/body wt./day. All patients received cotrimoxazole prophylaxis up till the first post-transplant year or lifelong. Valganciclovir prophylaxis was given for 6 months for CMV high-risk recipients.
Statistical analysis was performed using computer software GraphPad Prism version 7 (GraphPad Software, 2365 Northside Dr., San Diego, CA 92108). Categorical data were analyzed by the Chi-square test. Continuous variables were analyzed by 't' test. The graft and patient survival rates were analyzed by using the Kaplan–Meier method. A P value <0.05 was considered statistically significant.
Results
Our study included 154 patients with PDs and 75 patients with SDs [Figure 1]. Basic demographic details of the recipient are shown in Table 1A and of donor in Table 1B. Both urinary creatinine clearance and measured GFR were significantly higher in the SD compared to the PD group [Figures 2 and 3]. Analysis of the outcome data of both the groups is shown in Table 2. There was no significant difference in acute rejection, graft loss, or patient loss between PD and SD groups. However, the follow-up period was significantly higher for PD group (P < 0.05). Overall graft survival, death-censored graft survival and patient survival were 58%, 62% and 84% at 13.4 years in PD group, and overall graft survival, death-censored graft survival and patient survival were 73%, 81% and 90% at 12.9 years in SD group. There was no significant difference between patient loss, mean survival, graft survival (uncensored), and death-censored graft survival between two groups [Figures 4–6]. The causes of graft and patient loss were summarized in Table 3.
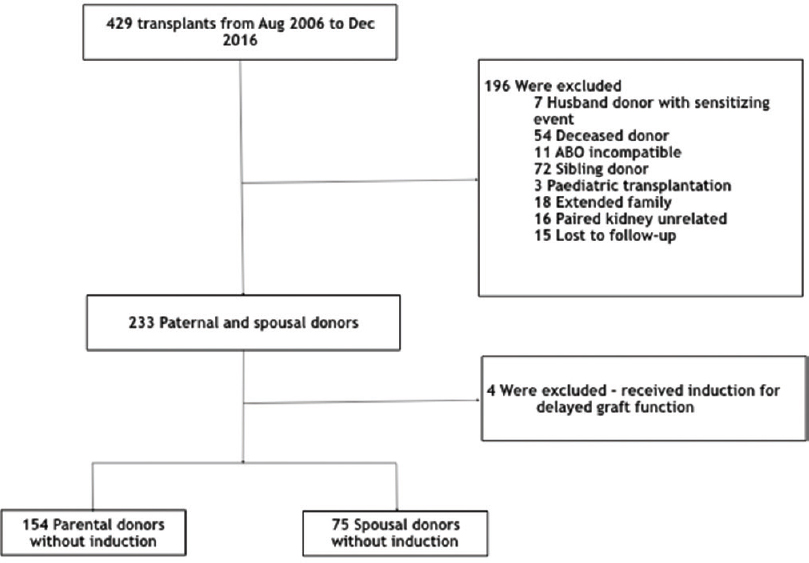
- Study cohort of parental and spousal donor transplantation
| Variable | PD group (n=154) | SD group (n=75) | Significance |
|---|---|---|---|
| Recipient’s Age (years) | 27.79±6.85 | 45.62±7.96 | P<0.001 |
| Male sex - no. (%) | 110 (71.4) | 75 (100) | ns |
| Native kidney disease - no. (%) | |||
| Chronic GN | 66 (43) | 22 (30) | ns |
| CTID | 26 (17) | 6 (8) | |
| Diabetic kidney disease | 23 (15) | 19 (26) | |
| Unclear aetiology | 28 (18) | 28 (38) | |
| HD Duration in months | 9.75±12.59 | 8.95±6.93 | ns |
| Induction | None | None | – |
| Maintenance immunosuppression (%) | Pred + Tac + MMF−88 | Pred + Tac + MMF−92 | – |
PD=Parental donor, SD=Spousal donor Chronic, GN=Chronic glomerulonephritis, CTID=Chronic tubulointerstitial nephritis, HD=Haemodialysis, Pred=Prednisolone, Tac=Tacrolimus, MMF=Mycophenolate mofetyl
| Variable | Parental donor group (n=154) | Spousal donor group (n=75) | Significance |
|---|---|---|---|
| Donor’s age (year) | 50.42±7.32 | 38.36±7.81 | P<0.001 |
| Female sex - no. (%) | 123 (79.8) | 75 (100) | ns |
| 24 h CrCl in mL/mt | 79.3±2.3 | 98.04±4.26 | P=0.0002 |
| mGFR by DTPA mL/min/1.73 m2 | 77.8±1.62 | 90.3±1.85 | P<0.0001 |
CrCl=Creatinine clearance, mGFR=Measured GFR, DTPA=Diethylenetriamine Pentaacetic Acid
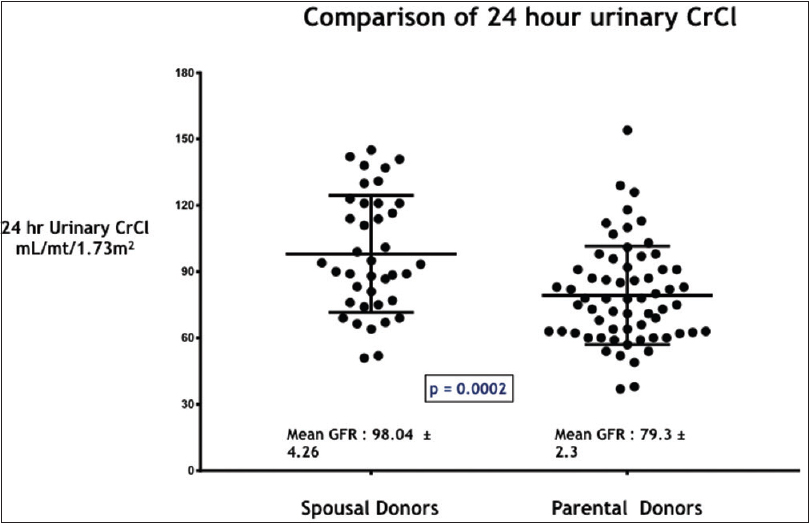
- Comparison of 24 hour urinary creatinine clearance (CrCl) in mL/mt/1.73m2 in the study groups – SD and PD
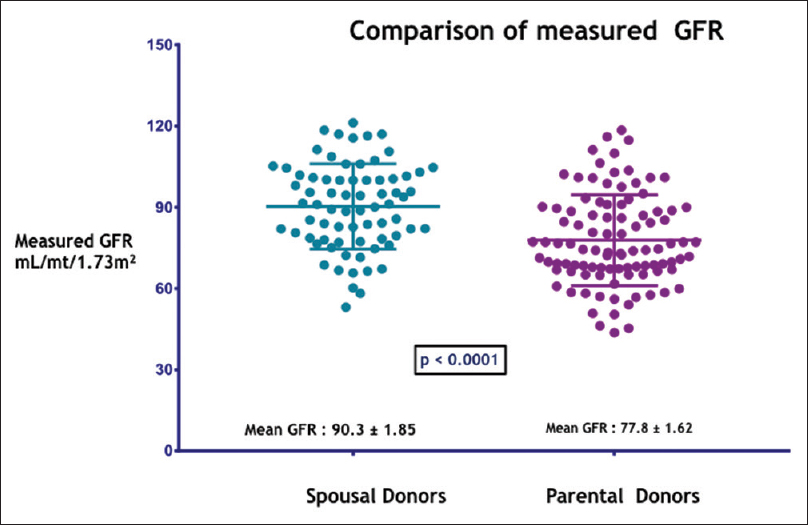
- Comparison of measured GFR by DTPA in mL/mt/1.73m2 in the study groups –SD and PD
| Variable | Parental donor group (n=154) | Spousal donor group (n=75) | Significance |
|---|---|---|---|
| Acute rejections in first year - no. (%) | 28 (18) | 13 (17) | P=0.85 |
| Acute cellular rejection (ACR) | 18 (11.7) | 7 (9.3) | ns |
| Acute antibody rejection (ABMR) | 5 (3.3) | 5 (6.7) | ns |
| Mixed rejection (ACR+ABMR) | 5 (3.3) | 1 (1.3) | ns |
| Overall graft loss - no. (%) | 34 (22) | 15 (20) | P=0.6 |
| Patient loss - no. (%) | 7 (4.5) | 6 (8) | P=0.23 |
| Death-censored graft loss - no. (%) | 28 (19) | 9 (13) | P=0.11 |
| Follow-up duration in days | 2613±83 | 2262±111 | P=0.01 |
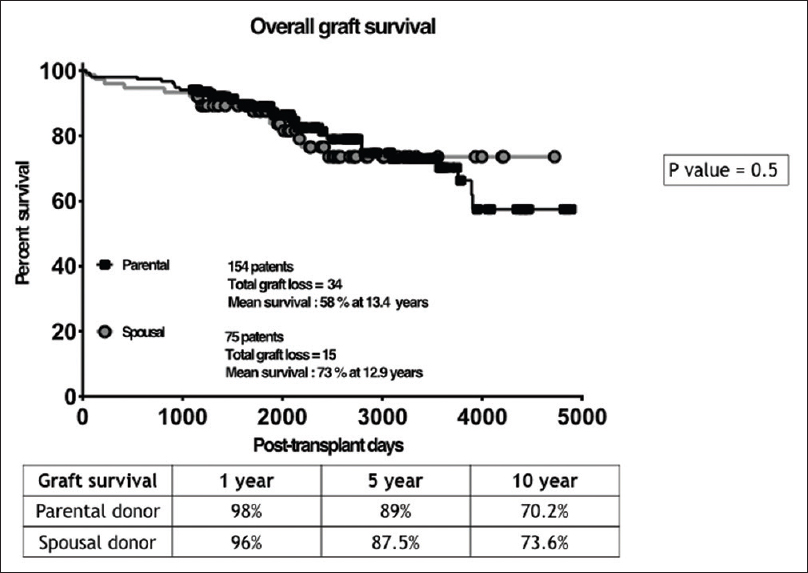
- Kaplan-Meier curves ccomparing the graft survival in the study groups –SD and PD
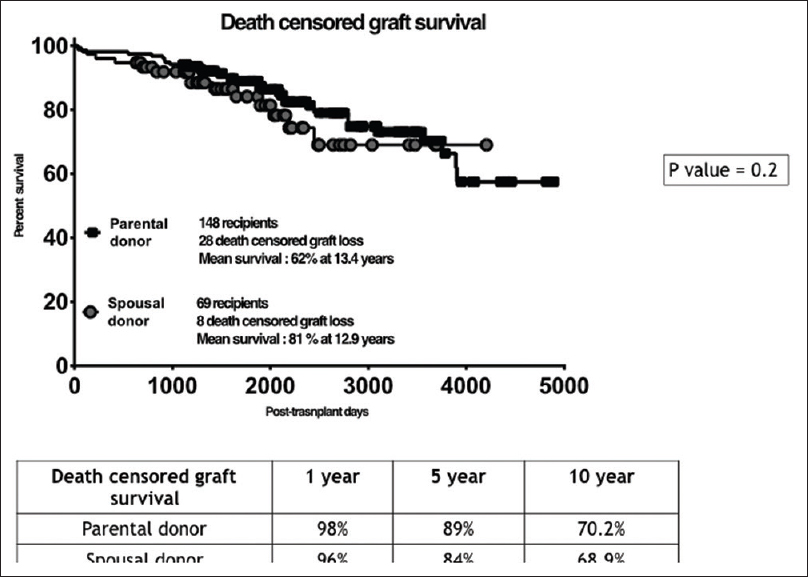
- Kaplan-Meier curves comparing the death censored graft survival in the study groups –SD and PD
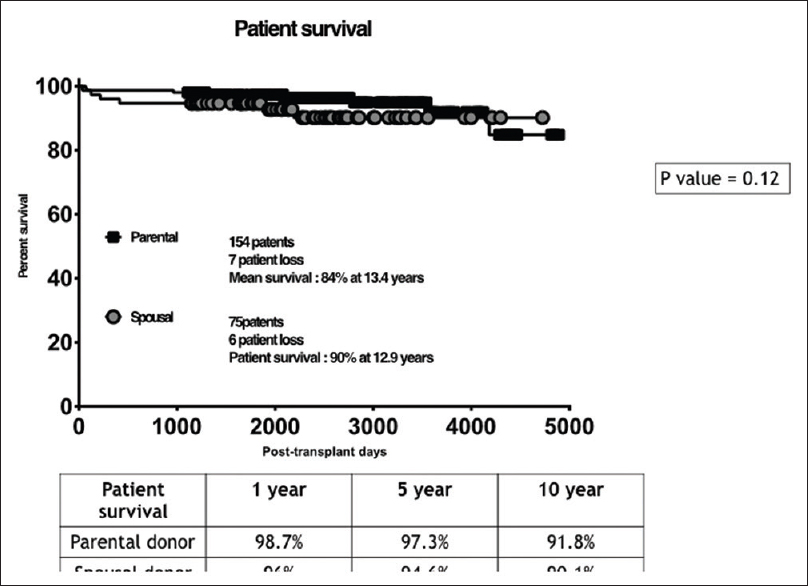
- Kaplan-Meier curves comparing the patient survival in the study groups –SD and PD
| Variable | Parental donor group (n=154) | Spousal donor group (n=75) |
|---|---|---|
| Death-censored graft loss - no. (%) | 28 (19) | 9 (13) |
| Causes for death censored graft loss | 1 - Graft thrombosis | 1 - BKVAN |
| 1 - BKVAN | 2 - Non-compliance rejection | |
| 3 - Non-compliance-rejection | 1 - Recurrent GN | |
| 6 - Recurrent GN/De novo GN | 4 - IFTA/Chronic rejection | |
| 17 - IFTA/Chronic rejection | ||
| Patient loss - no. (%) | 7 (4.5) | 6 (8) |
| Causes for patient loss | 1 - CMV, Sepsis | 1 - Aspergillus pneumonia |
| 1 - Neutropenic sepsis | 1 - Mucormycosis | |
| 1-- Acute pancreatitis | 1 - Mesenteric artery thrombosis - sepsis | |
| 2 - Sepsis, unclear source | 1 - Sepsis, unclear source | |
| 2 - Acute coronary syndrome | 2 - Acute coronary syndrome |
BKVAN=BK Virus-associated nephropathy, GN=Glomerulonephritis, CMV=Cytomegalovirus
Discussion
In the present study, we evaluated the outcomes of ILR transplant recipients who have received related PDs and SDs without any induction IS. Our study results showed that SDs were significantly younger and have significantly higher urinary creatinine clearance and measured GFR compared to the PD group. There was no significant difference in acute rejection, graft loss, patient loss, mean survival, graft survival (uncensored), or death-censored graft survival between two groups. To the best of our knowledge, this is the first study from India comparing the long-term outcome (>10 years) of ILR transplant recipients who have received PD and SD with a similar IS protocol. Our study results are not in agreement with most of the previously published studies that reported higher incidence of acute allograft rejection among SD group when compared with the PD group.[91012] Fuller et al. explored the risk factors for early rejection and its impact on living-related versus living unrelated kidney transplants (spouse [51.6%], friend [38.3%] or an in-law) over 5 years.[13] They found that the percentage of patients with early rejection and severe rejection was significantly higher among the living-unrelated kidney transplants group that includes SD, and the 1-year rejection rate was significantly lower in living-related kidney transplants group. However, the study failed to show any significant difference in overall patient survival, graft survival, and measured creatinine levels between the two groups.
A recent Indian study explored the outcomes of SD with RD kidney transplantation for 2 years. Similar to our study there was a significant difference in the age between SD and RD groups with SD group significantly younger.[11] The study also found that acute rejection rates and death-censored deranged graft function were significantly higher in the RD group compared to the SD group. However, a higher proportion of patients in the SD group received induction when compared with the RD group. In our study, none of the patients received induction IS. We found no difference in acute rejection rates and death-censored graft survival between SD and PD groups.
Our study results question the need for induction IS among ILR transplant recipients with SDs. Our study failed to show a significantly higher rate of acute rejection in the SD group. Though the currently prevailing clinical practice recommends initial immunosuppressive regimen in kidney transplant recipients especially in the context of SD, the previous studies evaluating induction IS showed contradictory findings. Two previous studies from India did not find a significant difference in acute rejection rates with or without induction therapy.[1415] The study by Fuller et al. also showed that the absence of induction therapy was not associated with a higher rejection rate.[13] However, while discussing the results, Mittal et al. argued that one of the potential reasons for a higher rate of rejection and poorer graft function at the end of follow-up among RD group when compared with SD group was that only a minority of patients in the RD group received induction IS.[11] Moreover, multiple systematic reviews and meta-analysis point to the fact that induction IS can significantly reduce the rate of acute rejection without significantly affecting all-cause mortality, malignancy or infection rates. Hence, the evidence-based protocol for induction IS in SD is not formed. KDIGO guidelines on IS has mentioned the inevitability of individualizing induction protocol in ILR group.[8] We suggest that among ILR transplant recipients with SD, induction IS can be avoided so that transplant costs can be reduced significantly especially in developing countries like India. However, patients should be screened with advanced pre-transplant cross-match techniques using newer and multiple platforms like flow-cytometer and Luminex as CDC-based crossmatch which is still used in most centers in developing countries is less sensitive in detecting circulating donor-specific antibodies.[16]
In conclusion, our study results showed that the outcomes of immunologically low-risk transplant recipients who have received PD and SD are similar and induction IS is not required among the SD groups provided they are immunologically profiled and characterized as true immunologically lower risk with newer and advanced histocompatibility methods using flow cytometer and Luminex technology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Causes of death in renal transplant recipients with functioning allograft. Indian J Nephrol. 2012;22:264-8.
- [Google Scholar]
- Chronic kidney disease in India: A clarion call for change. Clin J Am Soc Nephrol. 2018;13:802-4.
- [Google Scholar]
- Transplantation of human organs and tissues Act-”Simplified”. Indian J Transplant. 2018;12:84-9.
- [Google Scholar]
- Spousal renal donor transplantation in Chinese subjects: A 10 year experience from a single centre. Nephrol Dial Transplant. 2004;19:203-6.
- [Google Scholar]
- the effects of age-adjusted marriage duration on graft outcomes in spousal donor kidney transplantation 2019
- High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333-6.
- [Google Scholar]
- KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int. 2010;77:299-311.
- [Google Scholar]
- Long-term outcomes of renal transplants from spousal and living-related and other living-unrelated donors: A single center experience. J Assoc Physicians India. 2012;60:24-7.
- [Google Scholar]
- The spouse as a donor in renal transplants. Saudi J Kidney Dis Transpl. 2006;17:77-81.
- [Google Scholar]
- Outcomes of spousal versus related donor kidney transplants: A comparative study. Indian J Nephrol. 2014;24:3-8.
- [Google Scholar]
- Comparison of long-term outcomes between spousal transplants and other living unrelated donor transplants: Single-center experience. Nephron Clin Pract. 2009;113:c241-9.
- [Google Scholar]
- Increased rejection in living unrelated versus living related kidney transplants does not affect short-term function and survival. Transplantation. 2004;78:1030-5.
- [Google Scholar]
- Comparison of azathioprine with mycophenolate mofetil in a living donor kidney transplant programme. Indian J Nephrol. 2011;21:258-63.
- [Google Scholar]
- Efficacy of Basiliximab induction in poorly matched living donor renal transplantation. Ind J Nephrol. 2013;23:409-12.
- [Google Scholar]
- Laboratory assessment of HLA antibodies circa 2006: Making sense of sensitivity. Transplant Rev. 2006;20:189-94.
- [Google Scholar]







