Translate this page into:
Predicting the Risk of Progression in Indian ADPKD Cohort using PROPKD Score – A Single-Center Retrospective Study
Address for correspondence: Dr. HC Sreenidhi, Department of Nephrology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India. E-mail: sreenidhi1024@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
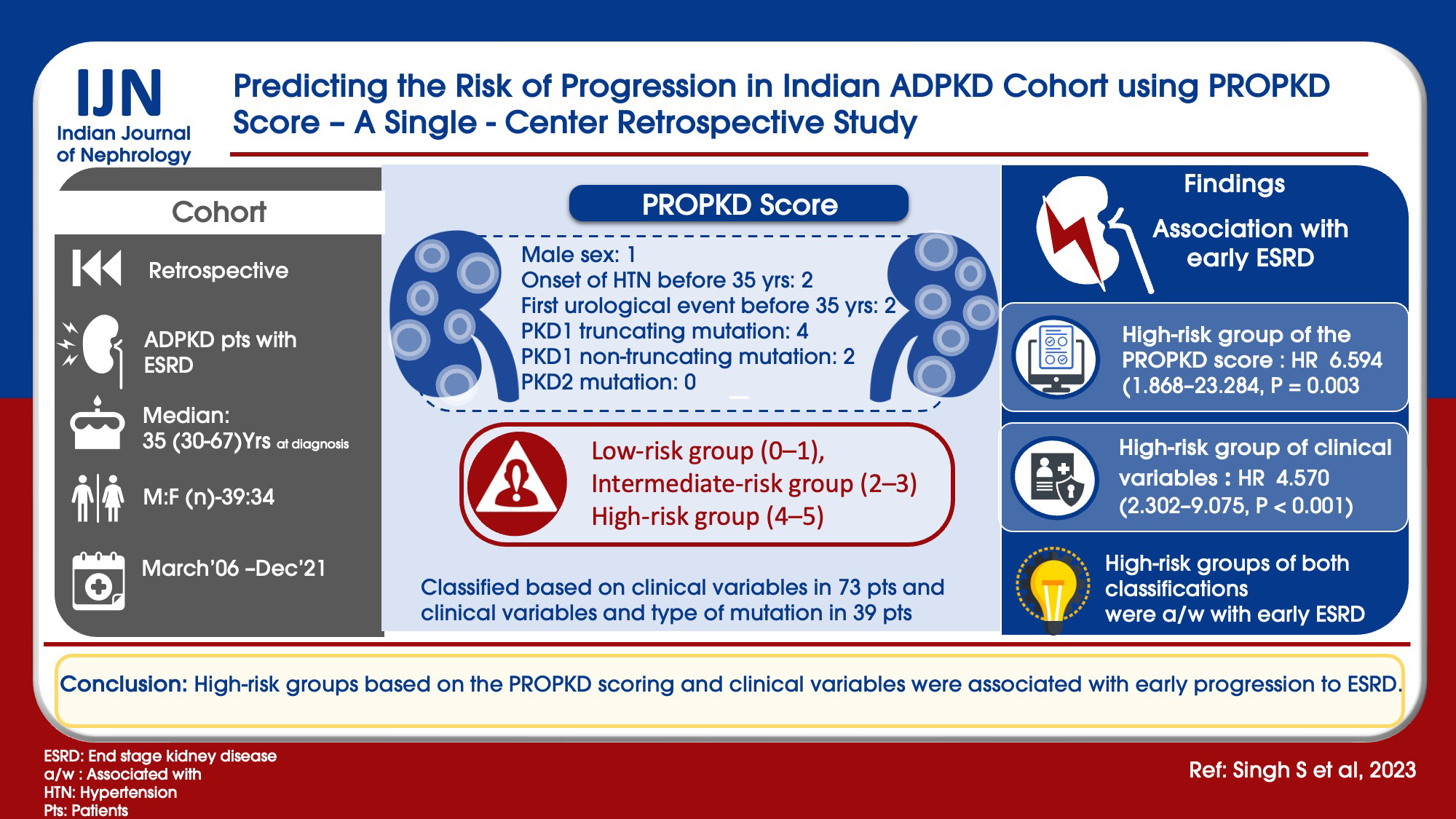
With the variable genotype–phenotype expression of autosomal dominant polycystic kidney disease (ADPKD) and availability of novel targeted therapies, it is important to find predictors for rapid progression. The PROPKD score, consisting of genetic and clinical parameters like sex, hypertension, and urological events, is a useful tool in predicting the risk of progression. This study was aimed to determine the risk of ADPKD progression in Indian patients using the PROPKD score.
Materials and Methods:
A retrospective study was done from 2006 to 2021. ADPKD patients with ESRD were included in the study. Scoring was done as per the PROPKD score as follows: male sex: 1, onset of hypertension before 35 years: 2, first urological event before 35 years: 2, PKD1 truncating mutation: 4, PKD1 non-truncating mutation: 2, and PKD2 mutation: 0. Two types of risk classifications were done as follows: (a) considering the clinical variables in all 73 patients (male sex, onset of hypertension before 35 years, and first urological event before 35 years), they were classified into three risk groups: low-risk group (0–1), intermediate-risk group (2–3), and high-risk group (4–5) and (b) considering the clinical variables and type of mutation in 39 patients, they were classified into three risk groups: low-risk group (0–3), intermediate-risk group (4–6), and high-risk group (7–9).
Results:
Total number of patients included was 73, with the median age at ESRD being 54 years. High-risk group of clinical variables with hazard ratio (HR) of 4.570 (2.302–9.075, P < 0.001) and high-risk group of the PROPKD score with HR of 6.594 (1.868–23.284, P = 0.003) were associated with early ESRD. High-risk groups of both classifications were associated with early ESRD.
Conclusion:
High-risk groups based on the PROPKD scoring and clinical variables were associated with early progression to ESRD.
Keywords
ADPKD
PKD1 and PKD2
progression to ESRD
PROPKD
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a commonly inherited kidney disease characterized by the growth of cysts in both kidneys, resulting in progressive renal failure.[1-3] ADPKD patients usually progress to end stage renal disease (ESRD) by the fifth or sixth decade of life.[1-3] The degree of decline in renal function is well correlated with cyst growth and total kidney volume (TKV).[4-7] PKD1 mutation is associated with a greater number of cysts and TKV and early progression to ESRD.[4-7]
With a better understanding of the pathogenesis of ADPKD, multiple targeted therapies have been found to be promising in retarding cyst growth and delaying the progression of renal failure.[8,9]
Due to variable genotype–phenotype expression, it is important to find predictors of rapid progression to renal failure. Mayo clinic predictor tool, which includes age- and height-adjusted TKV, is widely accepted.[10] The Predicting Renal Outcome in Polycystic Kidney Disease (PROPKD) score is an alternative tool established in the GENKYST cohort which uses a combination of genetics and clinical parameters like sex, hypertension, and urological events.[11]
In developing countries like India where there is poor accessibility to genetic studies and higher cost for radiology services, clinical parameters are important tools in predicting the risk of ADPKD progression. The present study aimed to determine the risk of ADPKD progression in Indian patients using the PROPKD score.
Materials and Methods
A retrospective observational study was conducted from March 2006 to December 2021 at the Department of Nephrology. Medical records were searched for ADPKD patients. Diagnosis of ADPKD was considered if there were presence of at least three (unilateral or bilateral) renal cysts in patients aged 15–39 years, two cysts in each kidney in patients aged 40–59 years, and four or more cysts in each kidney in patients aged ≥60 years[12] with or without a family history of ADPKD. ADPKD patients with ESRD (estimated glomerular filtration rate [eGFR] <15 ml/min/1.7 m2) were included in the study. Patients with a lack of clinical data, the onset of ESRD before 30 years of age, and non-cystic causes of CKD were excluded. Clinico-epidemiological data was collected from hospital records. Serum creatinine values were noted at diagnosis and follow-up from clinical records. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD EPI) equation[13] using serum creatinine, age, sex, and race. Staging of chronic kidney disease (CKD) was done as per the Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines considering eGFR.[14] After excluding patients who had ESRD at presentation, the rate of eGFR decline/year was calculated in the remaining patients by noting eGFR from the hospital records at yearly interval until eGFR was <15 ml/min/1.7 m2 or initiation of renal replacement therapy (RRT). Information on the type of mutation in patients who had undergone molecular analysis previously[15] was collected from the Centre for Genetic Disorders. Mutations were analyzed by Sanger sequencing.
Hypertension was defined as blood pressure of ≥140/90 mmHg or patients on antihypertensive therapy. Hemorrhagic events (cyst hemorrhage/gross hematuria), cyst infection, or flank pain related to cyst were considered as the urological events.[11] Scoring was done as per the PROPKD score as follows:[11] male sex: 1 point, the onset of hypertension before 35 years: 2 points, first urological event before 35 years: 2 points, PKD1 truncating mutation: 4 points, PKD1 non-truncating mutation: 2 points, and PKD2 mutation: no points. Since mutational data was available only in 39 patients, two types of risk classifications were done.
-
Considering the clinical variables in all 73 patients (male sex, onset of hypertension before 35 years, and first urological event before 35 years), they were classified into three risk groups: low-risk group (0–1), intermediate-risk group (2–3), and high-risk group (4–5).
-
Considering the clinical variables and type of mutation in 39 patients, they were classified into three risk groups: low-risk group (0–3), intermediate-risk group (4–6), and high-risk group (7–9).
Figure 1 shows the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) diagram of the study design.

- Flow chart of the study design. ADPKD = autosomal dominant polycystic kidney disease
Statistical analysis
Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) software version 28.0.0.0 (190). Continuous variables were expressed as median (range). Categorical variables were expressed as a percentage. Time to onset of ESRD was analyzed using Kaplan–Meier analysis with log-rank test for comparison. Components of the PROPKD score and risk groups for the onset of ESRD were analyzed by Cox proportional hazard models. The distribution of quantitative variables among the risk groups was analyzed using an independent Kruskal–Wallis test. P values < 0.05 were considered statistically significant.
Results
The total number of patients included was 73, with the median age at diagnosis of ADPKD being 35 years (range 30–67 years). There were 39 males (53.42%), and 51 patients (69.86%) had a family history of ADPKD. Eight patients (11%) were diabetic. Forty-three patients (58.9%) had onset of hypertension before 35 years of age. Twenty-nine patients (39.7%) had the first urological event before 35 years of age. There were two (2.7%) in stage I, four (5.5%) in stage II, 17 (23.3%) in stage III, 31 (42.5%) in stage IV, and 19 (26%) patients in stage V CKD. Molecular analysis was available for 39 (53.4%) patients. Twenty-six (66.7%) had PKD1 non-truncating mutation, seven (17.9%) had PKD1 truncating mutation, and six (15.4%) had PKD2 mutation. The median age of the study cohort at ESRD was 54 years (range 42–70 years). Characteristics of the study cohort are shown in Table 1.
| Characteristics | Value |
|---|---|
| Patients (n) | 73 |
| Median age at ADPKD diagnosis (years) | 35 (30-67) |
| Male: female (n) | 39:34 |
| Median serum creatinine (mg/dl) at diagnosis | 2.5 (1-12.5) |
| Median eGFR (ml/min/1.73 m2) at diagnosis | 26.29 (3.74-100.55) |
| Family history of ADPKD (n) | 51 (69.86%) |
| Diabetes mellitus | 8 (11%) |
| Hypertension before 35 years (n) | 43 (58.9%) |
| First urological event before 35 years (n) | 29 (39.7%) |
| Median age at ESRD (years) | 54 (42–70) |
| CKD stages (n) | 74 (100%) |
| Stage I | 2 (2.7%) |
| Stage II | 4 (5.5%) |
| Stage III | 17 (23.3%) |
| Stage IV | 31 (42.5%) |
| Stage V | 19 (26%) |
| Molecular analysis (n) | 39 (53.4%) |
| PKD1 non-truncating mutation | 26 (66.7%) |
| PKD1 truncating mutation | 7 (17.9%) |
| PKD2 mutation | 6 (15.4%) |
ADPKD=autosomal dominant polycystic kidney disease, eGFR=estimated glomerular filtration rate
On Kaplan–Meier analysis, the median age at ESRD was 52 years in males and 58 years in females (log-rank P = 0.013), the median age at ESRD was 51 years in those with onset of hypertension before 35 years and 60 years in those with onset of hypertension after 35 years (log-rank P < 0.001), and the median age at ESRD was 53 years in those with the first urological event before 35 years and 56 years in those with the first urological event after 35 years (log-rank P = 0.012) [Figure 2a–c].
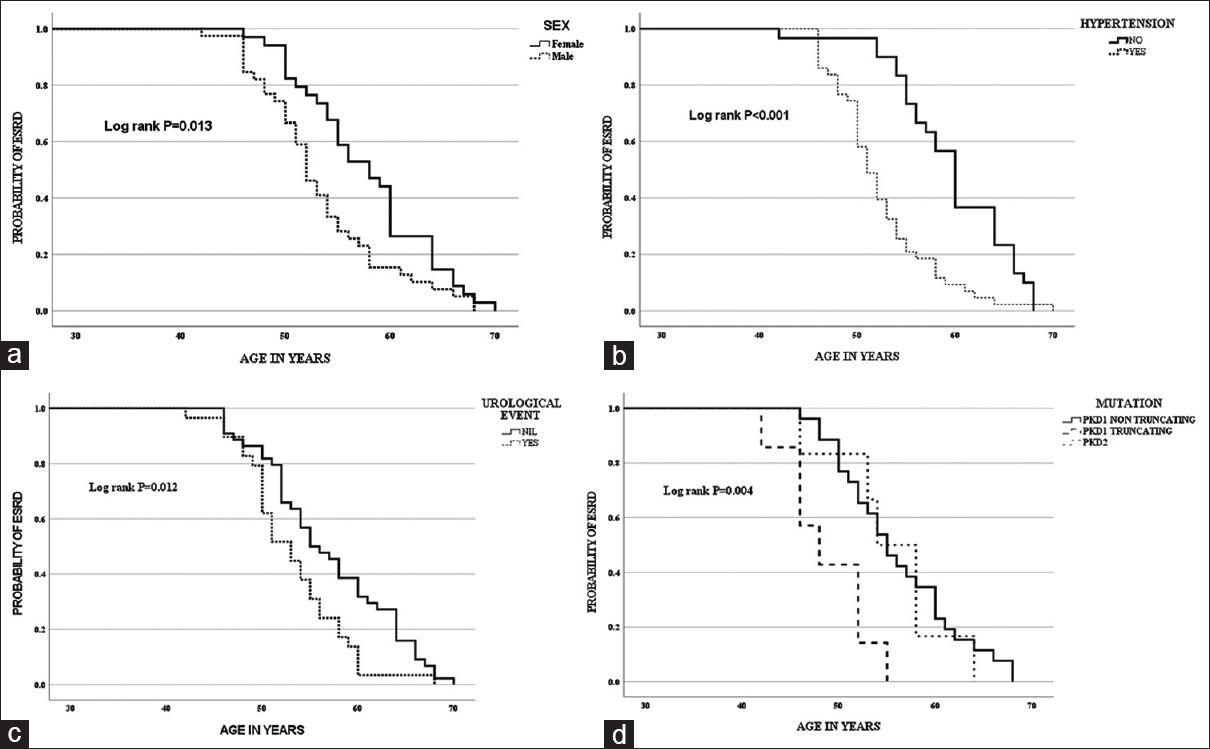
- (a) Kaplan–Meier curve for the onset of ESRD with sex. (b) Kaplan–Meier curve for the onset of ESRD with hypertension before 35 years. (c) Kaplan–Meier curve for the onset of ESRD with the first urological event before 35 years. (d) Kaplan–Meier curve for the onset of ESRD with the type of mutation
On univariate Cox regression analysis, male sex with hazard ratio (HR) of 1.722 (1.076–2.755, P = 0.023), onset of hypertension before 35 years with HR of 2.749 (1.677–4.506, P < 0.001), and first urological event before 35 years with HR of 1.779 (1.093–2.895, P = 0.02) were associated with early onset of ESRD. On multivariate Cox regression model, onset of hypertension before 35 years with HR of 2.486 (1.486–4.159, P < 0.001) and first urological event before 35 years with HR of 1.802 (1.099–2.954, P = 0.02) were associated with early onset of ESRD [Table 2].
| Hazard ratio | 95% Confidence interval | P | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Univariate Cox regression model (n=73) | ||||
| Sex | ||||
| Male (n=39) | 1.722 | 1.076 | 2.755 | 0.023 |
| Female (n=34) | ||||
| Hypertension before 35 years | ||||
| Yes (n=43) | 2.749 | 1.677 | 4.506 | <0.001 |
| No (n=30) | ||||
| Urological event before 35 years | ||||
| Yes (n=29) | 1.779 | 1.093 | 2.895 | 0.02 |
| No (n=44) | ||||
| Multivariate Cox regression model (n=73) | ||||
| Sex | ||||
| Male (n=39) | 1.484 | 0.917 | 2.401 | 0.108 |
| Female (n=34) | ||||
| Hypertension before 35 years | ||||
| Yes (n=43) | 2.486 | 1.486 | 4.159 | <0.001 |
| No (n=30) | ||||
| Urological event before 35 years | ||||
| Yes (n=29) | 1.802 | 1.099 | 2.954 | 0.02 |
| No (n=44) | ||||
In the subset of patients in whom genetic testing was done, Kaplan–Meier analysis showed that the median age at ESRD was 48 years with PKD1 truncating mutation, 54 years with PKD1 non-truncating mutation, and 55 years with PKD2 mutation (log-rank P = 0.004) [Figure 2d]. On Cox regression analysis, PKD1 truncating mutation was associated with early ESRD with HR of 3.466 (1.099–10.929, P = 0.034). On multivariate Cox regression model consisting of clinical and molecular variables, onset of hypertension before 35 years with HR of 3.024 (1.409–6.488, P = 0.005) and PKD1 truncating mutation with HR of 4.732 (1.786–12.536, P = 0.002) were associated with early ESRD [Table 3].
| Hazard ratio | 95% Confidence interval | P | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Cox regression model (n=39) | ||||
| PKD2 mutation (reference) n=6 | 0.013 | |||
| PKD1 non-truncating mutation n=26 | 0.872 | 0.354 | 2.151 | 0.766 |
| PKD1 truncating mutation n=7 | 3.466 | 1.099 | 10.929 | 0.034 |
| Multivariate Cox regression model (n=39) | ||||
| Sex | ||||
| Male (n=23) | 0.688 | 0.315 | 1.505 | 0.349 |
| Female (n=16) | ||||
| Hypertension before 35 years | ||||
| Yes (n=23) | 3.024 | 1.409 | 6.488 | 0.005 |
| No (n=16) | ||||
| Urological event before 35 years | ||||
| Yes (n=15) | 1.356 | 0.659 | 2.787 | 0.408 |
| No (n=24) | ||||
| PKD2 mutation (reference) | ||||
| n=6 | 0.007 | |||
| PKD1 non-truncating mutation | ||||
| n=26 | 1.044 | 0.415 | 2.625 | 0.927 |
| PKD1 truncating mutation | ||||
| n=7 | 4.732 | 1.786 | 12.536 | 0.002 |
Outcome in risk groups based on clinical variables
On Kaplan–Meier analysis, median age at ESRD was 50 years in high-risk group, 54 years in intermediate-risk group, and 60 years in low-risk group (log-rank P < 0.001) [Figure 3a]. On Cox regression model analysis, intermediate-risk group with HR of 2.061 (1.169–3.636, P = 0.012) and high-risk group with HR of 4.570 (2.302–9.075, P < 0.001) were associated with early ESRD [Table 4].
| Hazard ratio | 95% Confidence interval | P | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Cox regression model (n=73) | ||||
| Risk groups (male=1, onset of hypertension before 35 years=2, and first urological event before 35 years=2) | ||||
| Low (0-1) | ||||
| n=21 | <0.001 | |||
| Intermediate (2-3) | ||||
| n=32 | 2.061 | 1.169 | 3.636 | 0.012 |
| High (4-5) | ||||
| n=20 | 4.570 | 2.302 | 9.075 | <0.001 |
| Cox regression model (n=39) | ||||
| Risk groups (male=1, onset of hypertension <35 years=2, first urological event <35 years=2, PKD1 truncating mutation=4, PKD1 non-truncating mutation=2, and PKD2 mutation=0) | ||||
| Low (0-3) | ||||
| n=12 | 0.013 | |||
| Intermediate (4-6) | ||||
| n=23 | 1.354 | 0.668 | 2.745 | 0.401 |
| High (7-9) | ||||
| n=4 | 6.594 | 1.868 | 23.284 | 0.003 |
Outcome in risk groups based on clinical variables and type of mutation
In the subset of patients in whom genetic testing was done, Kaplan–Meier analysis showed that the median age at ESRD was 46 years in high-risk group, 54 years in intermediate-risk group, and 55 years in low-risk group (log-rank P = 0.003) [Figure 3b]. On Cox regression model analysis, high-risk group with HR of 6.594 (1.868–23.284, P = 0.003) was associated with early ESRD [Table 4].
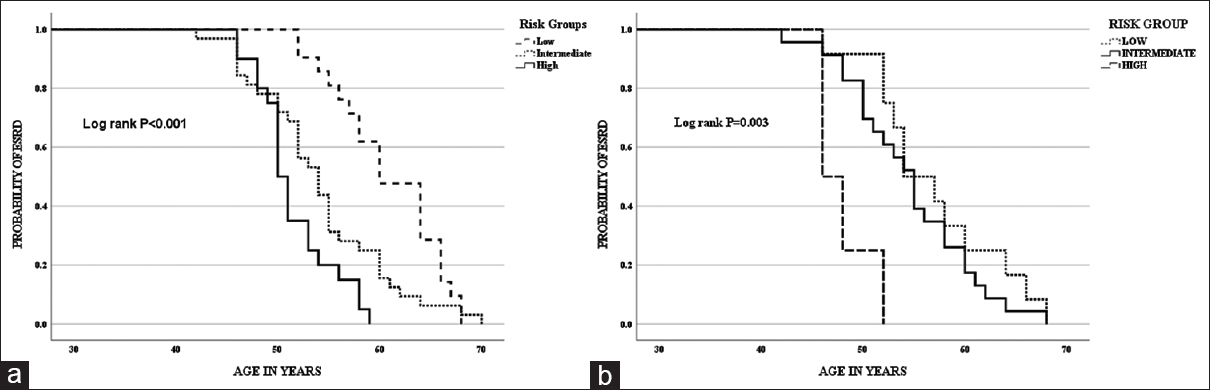
- (a) Kaplan–Meier curve for the onset of ESRD with risk groups considering clinical variables – low-risk group (0–1), intermediate-risk group (2–3), and high-risk group (4–5). (b) Kaplan–Meier curve for the onset of ESRD with risk groups considering clinical variables and type of mutation – low-risk group (0–3), intermediate-risk group (4–6), and high-risk group (7–9)
The distribution of age at ESRD was significant between the risk groups with early ESRD in high-risk group [Figure 4a and b].
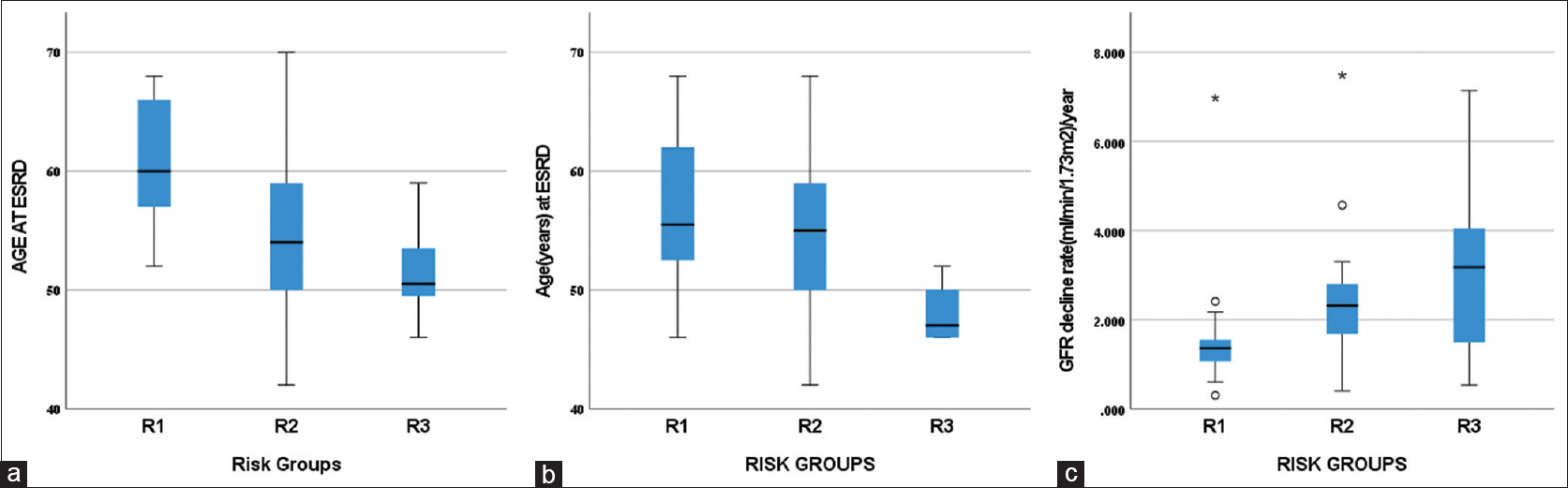
- (a) Distribution of age at ESRD among the risk groups based on clinical variables (R1- low risk, R2- intermediate risk, R3- high risk, P < 0.001 with independent Kruskal–Wallis test). (b) Distribution of age at ESRD among the risk groups based on clinical variables and type of mutation (R1- low risk, R2- intermediate risk, R3- high risk, P = 0.039 with independent Kruskal–Wallis test). (c) Distribution of rate of eGFR decline/year among the risk groups based on clinical variables (R1- low risk, R2- intermediate risk, R3- high risk, P = 0.003 with independent Kruskal–Wallis test). eGFR = estimated glomerular filtration rate
After excluding 19 patients of CKD stage V, the degree of eGFR decline/year was analyzed in the remaining 52 patients. The degree of eGFR decline was not found different in the risk groups based on clinical variables and mutation type as all four patients in the high-risk group were stage V at diagnosis. The median time to ESRD was 8 years (range 1–22 years). Median eGFR decline/year was 1.94 ml/min/1.73 m2/year. The distribution of eGFR decline/year was significant between the risk groups with greater decline found in the high-risk group [Figure 4c].
Discussion
This retrospective study was done to determine the risk of progression of ADPKD in Indian patients using the PROPKD score. The age at onset of ESRD in ADPKD varies significantly with the gene and type of mutation. PKD1 is the most commonly involved gene, followed by PKD2.[16-18] The median age at ESRD with PKD1 mutation varies from 53 to 58 years, whereas with PKD2 mutation, the median age varies from 68 to 79 years.[16-18] Consistent with these findings, in our cohort, PKD1 mutation was associated with early onset of ESRD with a median age of 54 years. PKD1 truncating mutation is associated with rapid progression of renal failure.[16] Similarly, we found patients with truncating PKD1 to have early onset of ESRD. Due to small number of patients with PKD2 and PKD1 truncating mutations in our study, it is difficult to comment on the median age at onset of ESRD with other ADPKD cohorts.
Gabow et al.,[19] in their retrospective cohort study of 580 ADPKD patients with 25 years of follow-up, reported male gender to have worse renal function. Similarly, another retrospective study of 1215 ADPKD patients by Johnson et al.[20] reported male gender to be associated with early onset of ESRD with a median age of 52 years. Similarly, in our cohort, males were associated with early onset of ESRD with a median age of 52 years, that is, 6 years earlier than females (median age 58 years).
Hypertension is common in the early stages of ADPKD with normal renal function. Marlais et al.,[21] in their systematic review and meta-analysis, reported prevalence of hypertension to be 20% in children with ADPKD. An analysis of data from 506 ADPKD adults showed that the patients who were hypertensive before 35 years of age developed ESRD 14 years earlier than the patients who were normotensive until 35 years of age.[20] In HALT-PKD trial,[22] rigorous blood pressure control in early ADPKD was associated with a slower increase in TKV. Ozkok et al.,[23] in their prospective observational study of 323 ADPKD patients for 100 months, reported hypertension as an independent risk factor of CKD progression. Consistent with these findings, in our cohort, the onset of hypertension before 35 years of age was associated with early progression to ESRD.
Johnson et al.[20] reported early ESRD (median age 49 years) in 128 ADPKD patients who had gross hematuria at age <30 years compared to 448 patients without gross hematuria (median age 59 years). The studies have shown increased frequency of urinary tract infection in ADPKD to be associated with rapid progression of renal failure. Schrier et al.,[24] in their systematic review and meta-analysis, reported PKD1 truncating mutation, male gender, early onset of hypertension, and early and frequent gross hematuria as significant predictors of rapid progression. Similarly, in our cohort, we found male sex, the onset of hypertension before 35 years of age, first urological event before 35 years of age, and PKD1 truncating mutation to be associated with early progression to ESRD.
In our study, in the multivariate Cox regression model of clinical variables, hypertension before 35 years of age and first urological event before 35 years of age were significantly associated with early onset of ESRD. In the multivariate Cox regression model of clinical variables and genetic analysis, onset of hypertension before 35 years of age and PKD1 truncating mutation were significantly associated with early onset of ESRD. Probably, because of the small sample size we could not get all variables significant on multivariate Cox regression.
However, consistent with the PROPKD study,[11] we found high-risk group based on clinical variables and type of mutation to be significantly associated with early onset of ESRD on Kaplan–Meier analysis and Cox regression. Interestingly, we found even the high-risk group based on clinical variables to be significantly associated with early onset of ESRD on Kaplan–Meier analysis and Cox regression. The eGFR decline/year in ADPKD varies from 0.5 to 4 ml/min/1.73 m2/year depending on the population, method of estimation, age, and type of mutations.[25,26] In our cohort, the mean eGFR decline of the high-risk group based on clinical variables was 3.09 ± 1.83 ml/min/1.73 m2/year, which is almost comparable with the eGFR decline for rapid disease progression (≥3 ml/min/1.73 m2/year).[27]
In the TEMPO3:4 trial[8] and REPRISE trial,[9] use of tolvaptan was associated with slower decline of renal function in early and later stages of ADPKD. With these novel targeted therapies, it is important to have a prognostic tool to detect patients with rapid decline who will benefit from therapy. Our study showed that the PROPKD score and its four components are useful in predicting the risk for rapid progression. We have shown that the risk groups based on clinical variables of PROPKD also predict rapid progression to ESRD, which can be used by clinicians to rationalize the use of therapeutic agents where data on mutation is not available. However, this needs to be validated in a larger population and in different ethnicities. We have shown that where genetic analysis is not available, early-onset hypertension and early onset of urological events are the markers of rapid progression. Conversely, where genetic analysis is available, most of the risk is explained by the genetic mutation and early-onset hypertension. Overall, this suggests routine measurement of blood pressure is to be encouraged in ADPKD. Early and aggressive control of hypertension is essential in ADPKD patients for delaying progression of renal failure.
The strength of our study is that we have demonstrated that the clinical parameters of the PROPKD score can be used to predict disease progression. We also report the common types of mutations seen in Indian ADPKD patients and their association with disease progression. Our study has the following limitations: it is a single-center study with a small sample size, and data on genetic mutation analysis was not available for all patients. We could not comment on the mutation with other ADPKD cohort due to the small sample size. We could not comment on the risk of progression using the Mayo clinic tool due to the lack of radiological data.
Conclusion
In a small cohort of 73 Indian ADPKD patients, the high-risk group based on the clinical components of the PROPKD score was associated with early progression to ESRD. In resource-poor settings, where there is a lack of access to genetic testing, risk groups based on the clinical variables of PROPKD can be used to predict the risk of progression of ADPKD.
Ethical approval
Institute ethics committee approval was taken (ethical code # ECR/526/Inst/UP/2014/RR-20/2258).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
All emeritus professors and past and present senior residents of Department of Nephrology, IMS, BHU Varanasi are acknowledged. Mrs. Sonam Raj, Department of Genetic Disorders, BHU Varanasi is also acknowledged.
References
- Epidemiology of autosomal-dominant polycystic kidney disease: An in-depth clinical study for south-western Germany. Nephrol Dial Transplant. 2013;28:1472-87.
- [Google Scholar]
- Board of the Working Group for Inherited Kidney Diseases of the European Renal Association and European Dialysis and Transplant Association. Rare inherited kidney diseases: Challenges, opportunities, and perspectives. Lancet. 2014;383:1844-59.
- [Google Scholar]
- Long-term trajectory of kidney function in autosomal-dominant polycystic kidney disease. Kidney Int. 2019;95:1253-61.
- [Google Scholar]
- Baseline total kidney volume and the rate of kidney growth are associated with chronic kidney disease progression in Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2018;93:691-9.
- [Google Scholar]
- Growth pattern of kidney cyst number and volume in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2019;14:823-33.
- [Google Scholar]
- Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407-18.
- [Google Scholar]
- Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377:1930-42.
- [Google Scholar]
- Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160-72.
- [Google Scholar]
- The PROPKD score: A new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27:942-51.
- [Google Scholar]
- Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20:205-12.
- [Google Scholar]
- A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-12.
- [Google Scholar]
- The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011;80:17-28.
- [Google Scholar]
- Mutational screening of PKD1 and PKD2 in Indian ADPKD patients identified 95 genetic variants. Mutat Res. 2020;821:111718.
- [Google Scholar]
- Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24:1006-13.
- [Google Scholar]
- Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol. 1997;8:1560-7.
- [Google Scholar]
- Linkage, clinical features, and prognosis of autosomal dominant polycystic kidney disease types 1 and 2. J Am Soc Nephrol. 1996;7:2142-51.
- [Google Scholar]
- Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;41:1311-9.
- [Google Scholar]
- Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol. 1997;8:1560-7.
- [Google Scholar]
- Hypertension in autosomal dominant polycystic kidney disease: A meta-analysis. Arch Dis Child. 2016;101:1142-7.
- [Google Scholar]
- Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255-66.
- [Google Scholar]
- Clinical characteristics and predictors of progression of chronic kidney disease in autosomal dominant polycystic kidney disease: A single center experience. Clin Exp Nephrol. 2013;17:345-51.
- [Google Scholar]
- Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol. 2014;25:2399-418.
- [Google Scholar]
- Patterns of kidney function decline in autosomal dominant polycystic kidney disease: A post hoc analysis from the HALT-PKD trials. Am J Kidney Dis. 2018;71:666-76.
- [Google Scholar]
- Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: A longitudinal study. Am J Kidney Dis. 2002;39:1127-34.
- [Google Scholar]
- An update on the use of tolvaptan for ADPKD: Consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders (WGIKD), the European Rare Kidney Disease Reference Network (ERKNet) and Polycystic Kidney Disease International (PKD-International) Nephrol Dial Transplant 2021:gfab312. doi: 10.1093/ndt/gfab312
- [Google Scholar]







