Translate this page into:
Prevalence of Asymptomatic COVID-19 Infection in Hemodialysis Patients and the Risk of Hypercoagulability: Should we Consider Routine Screening?
Address for correspondence: Prof. Haitham Ezzat, Assistant Professor of Nephrology, Department of Internal Medicine, Ain Shams University, Faculty of Medicine, Ramsis Street 38, Abbasia, Postal Code: 11566, Cairo, Egypt. E-mail: haitham_ezzat@hotmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Coronavirus disease 2019 (COVID-19) has become a pandemic in late 2019. Its clinical presentation varies from asymptomatic infection to severe respiratory failure. Infection control strategies to minimize the risk of transmission of COVID-19 in end-stage renal disease (ESRD) patients receiving in-center hemodialysis (HD) have been implemented. Development of humoral response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in adult patients with ESRD receiving HD has not been sufficiently reported.
Methods:
A total of 179 asymptomatic HD patients undergoing regular HD were screened for COVID-19 infection. Infection with SARS-CoV-2 was confirmed through a real-time reverse transcription polymerase chain reaction assay of nasopharyngeal swab specimens. They were classified into positive and negative groups according to the results of PCR.
Results:
Of the 179 asymptomatic patients, we found that 23 patients (12.8%) were positive for COVID-19. Their mean age was 45.61 ± 13.38 years. There was a significant difference between both groups regarding C-reactive protein, lymphocytes, and platelet counts (P < 0.001). Also, TAT (thrombin–antithrombin complex) and D-dimer levels were significantly increased among the positive group (11.47 ± 1.51 vs. 7.53 ± 1.64 mcq/L, P < 0.001; 1171.52 ± 267.6 vs. 542.76 ± 107.06 ng/mL, P < 0.001, respectively).
Conclusion:
Asymptomatic SARS-CoV-2 infection is detected in HD patients. They carry the risk of hypercoagulability complications. We need more strict infection control measures and proactive diagnosis to limit the spread of the infection and lethal thromboembolic complications.
Keywords
Asymptomatic infection
COVID-19
ESRD
hemodialysis
hypercoagulability
Introduction
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started in Wuhan in December 2019 and has already spread worldwide.[1] Hemodialysis (HD) patients have higher mortality than the general population. Infections are the second common cause of death in these patients.[2] HD patients may be at higher risk of infection with COVID-19 because of the need to go to the dialysis centers at an average of three times a week and frequent hospital admissions. This makes the prevention and control of infectious diseases more challenging in HD patients than that in the general population.[3] Till now, there are limited data on HD patients with COVID-19. One study showed that 37 of the 230 HD patients enrolled were infected with SARS-CoV-2, among whom six died (crude mortality rate 16.2%).[4] This suggests that it is imperative to take effective protective interventions to contain the spread of SARS-CoV-2 in dialysis units. Nephrology societies developed the guidelines for the prevention and limitation of COVID-19 infection in HD units.[5] But the drawback is that COVID-19 symptoms appear almost after an incubation period of about 6 days.[6] However, the clinical presentation is varied from asymptomatic presentation to severe disease with pneumonia and acute respiratory distress syndrome.[7] Asymptomatic transmission of SARS-CoV-2 is an important issue in the control of this pandemic as it appears to be a major source of contagion. Additionally, between 18% and 30%of SARS-CoV-2 may be asymptomatic. Asymptomatic carriers can also transmit the disease and intensify the pandemic.[8]
Methods
This cross-sectional study was performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study. This study was conducted on 179 prevalent HD patients. Patients with malignancies, countercurrent infections, active collagen disease, and patients prone to surgical procedure lately were excluded from the study. All included patients were asymptomatic for COVID-19 according to “the new coronavirus pneumonia prevention and control program published by the national health commission of China.”[9] They were tested for coronavirus using polymerase chain reaction (PCR) by nasopharyngeal sampling; infection with SARS-CoV-2 was confirmed through a real-time reverse transcription polymerase chain reaction (RT-PCR) assay of nasopharyngeal swab specimens following the routine screening. RT-PCR was carried out as per the Public Health England guidelines using certification-marked assays with primers directed to the nucleocapsid or RNA (ribonucleic acid)-dependent RNA polymerase genes. According to the results of PCR, the patients were classified into two groups:
-
Group 1: Including 23 asymptomatic prevalent HD patients tested positive for COVID-19
-
Group 2: Including 156 asymptomatic prevalent HD patients tested negative for COVID-19
All participants were subjected to full history including the etiology of end-stage renal disease (ESRD), the duration of HD, and the associated hepatitis C virus (HCV) infection. Hematology workup was done; hemoglobin (Hb) level, lymphocytic count, and platelet count were measured, and also C-reactive protein (CRP) was measured. The blood samples were collected before the dialysis session, stored in sodium citrate anticoagulated tubes, and analyzed within 30 minutes after collection. As hypercoagulability is a common complication in COVID-19 infection and many reported cases died due to hypercoagulability complications as pulmonary embolism, in addition to the previously listed investigations we also assessed the hypercoagulability state using D-dimer and thrombin–antithrombin complex (TAT) levels and compared the results between both groups. TAT is measured by enzyme immunoassay using a sandwich technique. The patient sample containing TAT is incubated with antibodies against thrombin, and the unbound constituents are removed by washing. Enzyme-conjugated antibodies to antithrombin are then added to the reaction, and the excess antibodies are removed by washing. The remaining (bound) enzymatic activity acts on a chromophore, and color development is proportional to the TAT in the sample. An additional group of 35 normal healthy subjects was added to our study design as a control group to compare the levels of TAT.
Statistical analysis
We used SPSS (Statistical Package for the Social Sciences) Version 24 to analyze the data. Categorical data were described in terms of frequencies and percentages. Numerical data were described in terms of means and standard deviations if normally distributed. Kolmogorov–Smirnov test was used to test the normality of data. Chi-square test was used to test the association between qualitative variables. Independent t-test was used to measure the statistical significance of the difference in means between both groups. Analysis of variance was used to test the statistical significance of the difference between more than two groups with the Bonferroni post hoc test. Pearson correlation was used to test the association between numerical variables. A receiver operating characteristics (ROC) curve was used to assess the effectiveness of TAT in the detection of hypercoagulability state in HD patients with COVID. In all statistical tests, P < 0.05 was considered statistically significant.
Results
This study was conducted on 179 prevalent HD patients. Their mean age was 43.4 ± 12.8 years. Most of them were males (57%). Of the 179 asymptomatic patients, 23 patients (12.8%) were tested positive for COVID-19. One aspect of the problem is that these patients had been not isolated, which means that they might have spread the infection for a period within the dialysis centers and within their families and the community. In this study, diabetes mellitus was the most common cause of ESRD (42.5%) followed by hypertension (22.3%). Fifty patients were HCV positive (27.9%). The median Hb level was 9.7 (9–10.2) g/dL. The median platelets, absolute lymphocyte counts, and CRP levels are shown in Table 1. The median level of D-dimer was 550 (480–660 ng/mL). On the other hand, the mean level of TAT was found to be 8.03 ± 2.1 mcq/L as shown in Table 1.
| Variable | n (%) |
|---|---|
| Age (years) | 43.4±12.8** |
| Gender | |
| Male | 102 (57)* |
| Female | 77 (43)* |
| Cause of ESRD | |
| Unknown | 26 (14.5)* |
| PCKD | 4 (2.2)* |
| HTN | 40 (22.3)* |
| DM | 76 (42.5)* |
| Glomerulonephritis | 15 (8.4)* |
| SLE | 16 (8.9)* |
| Urological cause | 2 (1.2)* |
| HCV+ve | 50 (27.9)* |
| Hb (g/dL) | 9.7 (9-10.2)*** |
| Platelets×109/L | 255 (190-316)*** |
| Absolute lymphocyte count×103µL | 2.1 (1.4-2.9)*** |
| CRP (mg/dL) | 8.4 (6.9-12)*** |
| SARS-CoV-2 PCR+ve | 23 (12.8)* |
| D-dimer (ng/mL) | 550 (480-660)*** |
| TAT (mcg/L) | 8.03±2.1** |
*Frequency (percentage). **Mean±standard deviation. ***Median (first quartile to third quartile) ESRD=end-stage renal disease; PCKD=polycystic kidney disease; HTN=hypertension; DM=diabetes mellitus; SLE=systemic lupus erythematosus; HCV=hepatitis C virus; +ve=positive; Hb=hemoglobin; CRP, C-reactive protein; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2; PCR=polymerase chain reaction; TAT=thrombin– antithrombin complex
Diagnosis of COVID-19
We found that there was no significant difference number of infected persons between the males and females (P = 0.618). Although positive COVID-19 patients were slightly older, this was statistically insignificant (P = 0.381). There was no significant difference between both groups regarding the Hb level (P = 0.403); in addition, we found no statistically significant association between being HCV positivity and COVID-19 positivity (P = 0.83). On the other hand, we found that positive COVID-19 HD patients had a highly significant lower platelet count than those who tested negative (P < 0.001). They also had significantly lower levels of lymphocytes compared with negative COVID-19 HD patients (P < 0.001). CRP levels were extremely high in positive COVID-19 patients (P < 0.001) as shown in Table 2. D-dimer levels were significantly higher in COVID-19 positive HD patients compared with those in the COVID-19 negative group (1171.52 ± 267.6 vs. 542.76 ± 107.06 ng/mL; P < 0.001) as shown in Figure 1. With respect to TAT levels, we found a statistically significant difference between both groups. COVID-19-positive HD patients had higher levels compared with COVID-19-negative patients (P < 0.001) as shown in Table 2 and Figure 2.
| Variable | SARS-CoV-2 PCR | P | |
|---|---|---|---|
| Positive | Negative | ||
| Age* (years) | 45.61±13.38 | 43.09±12.78 | 0.381 |
| Gender** | |||
| Male | 12 (52.2) | 90 (57.7) | 0.618 |
| Female | 11 (47.8) | 66 (42.3) | |
| Causes of ESRD** | |||
| PCKD | 1 (4.3) | 3 (1.9) | 0.966 |
| DM | 11 (47.8) | 65 (41.9) | |
| GN | 1 (4.3) | 14 (9) | |
| HTN | 5 (21.7) | 35 (22.6) | |
| SLE | 2 (8.7) | 14 (9) | |
| Unknown | 3 (13) | 23 (14.8) | |
| Urological | 0 | 1 (0.6) | |
| Duration of dialysis* (years) | 5.7±4.1 | 5±2.9 | 0.306 |
| Hb level* (g/dL) | 9.8±1 | 9.7±1 | 0.403 |
| Absolute lymphocyte count* × 103 µL | 0.92±0.15 | 2.45±0.95 | <0.001 |
| CRP* (mg/dL) | 52.5±12.49 | 9.3±4.9 | <0.001 |
| Platelets* × 109/L | 107.26±29.54 | 275.52±79.05 | <0.001 |
| HCV** | |||
| Positive | 6 (26.1) | 44 (28.2) | 0.833 |
| Negative | 17 (73.9) | 112 (71.8) | |
| D-dimer* (ng/mL) | 1171.52±267.6 | 542.76±107.06 | <0.001 |
| TAT* (µg/L) | 11.47±1.51 | 7.53±1.64 | <0.001 |
*Mean±standard deviation. **Frequency (percentage). COVID-19=coronavirus disease 2019; PCR=polymerase chain reaction; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2; PCKD=polycystic kidney disease; HTN=hypertension; DM=diabetes mellitus; SLE=systemic lupus erythematosus; HCV=hepatitis C virus; Hb=hemoglobin; CRP, C-reactive protein; TAT=thrombin–antithrombin complex
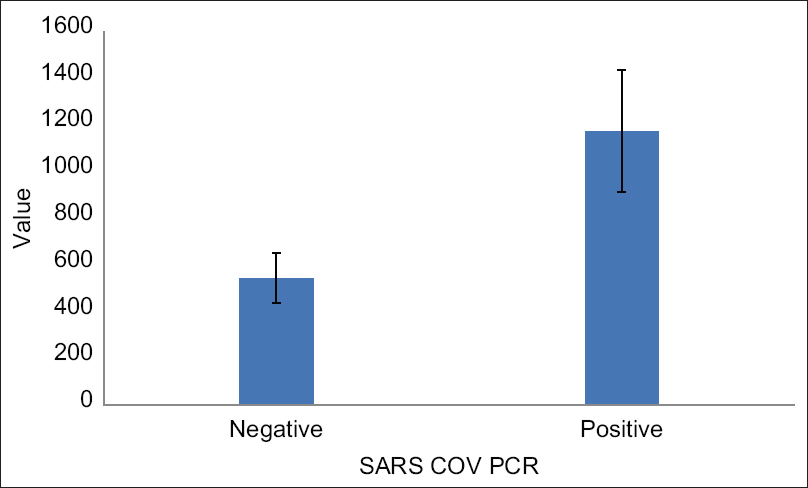
- Demonstrating the difference in D-dimer levels between COVID-19-positive and COVID-19-negative hemodialysis patients. SARS-CoV = severe acute respiratory syndrome coronavirus; PCR = polymerase chain reaction

- Difference in thrombin–antithrombin complex (TAT) levels between COVID-19-positive and COVID-19-negative hemodialysis patients. SARS-CoV = severe acute respiratory syndrome coronavirus; PCR = polymerase chain reaction
D-Dimer
On subgroup analysis for COVID-19 positive patients (23 patients), we found that there was a weak correlation between D-dimer levels and age, Hb level, and duration of dialysis. However, none of these was statistically significant (P = 0.41, 0.99, and 0.49, respectively) as shown in Table 3. On the other hand, there was a negative correlation between D-dimer levels and platelet count and lymphocytic count. In addition, there was a positive correlation between D-dimer levels and CRP levels. All these correlations were also statistically insignificant (P = 0.05, 0.18, and 0.07, respectively) as shown in Table 3.
| Correlation coefficient | P | |
|---|---|---|
| Age (years) | 0.18* | 0.41 |
| Gender | ||
| Male | 1172.08±220.922,, | 0.992 |
| Female | 1170.91±322.256,, | |
| Duration of dialysis (years) | 0.15* | 0.49 |
| HCV | ||
| Positive | 1138.3±350.6,, | 0.73 |
| Negative | 1183.2±243.9,, | |
| Hb (g/dL) | −0.003* | 0.99 |
| Platelets | −0.405* | 0.05 |
| Lymphocytes | −0.29* | 0.18 |
| CRP | 0.39* | 0.07 |
*Pearson correlation, , Mean±standard deviation. COVID-19=coronavirus disease 2019; HCV=hepatitis C virus; Hb=hemoglobin; CRP, C-reactive protein
TAT levels
On subgroup analysis on COVID-19-positive HD patients (23 patients), we found that neither the CRP level nor the lymphocytic count was significant determinants of TAT levels in those patients (P = 0.47 and 0.3, respectively). Platelet count was the significant indicator of TAT level in COVID-19-positive ESRD patients as shown in Table 4. On comparing the HD patients’ group, whether COVID-19 positive or negative, with healthy individuals group consisting of 35 subjects who were age and sex matched, we found a statistically significant difference between them with regard to TAT levels and D-dimer levels as shown in Table 5. On applying ROC curve analysis, we found that TAT can be an excellent method to assess the hypercoagulability state in asymptomatic HD patients liable for COVID-19 infection (area under the curve = 0.966; 95% confidence interval: 0.935–0.988; P < 0.001) as shown in Figure 3.
| Correlation coefficient | P | |
|---|---|---|
| Age (years) | 0.14* | 0.53 |
| Gender | ||
| Male | 11.43±1.54,, | 0.91 |
| Female | 11.5±1.55,, | |
| Duration of dialysis (years) | −0.09* | 0.67 |
| HCV | ||
| Positive | 11.3±1.72,, | 0.76 |
| Negative | 11.52±1.48,, | |
| Hb (mg/dL) | 0.16* | 0.46 |
| Platelets | −0.43* | 0.04 |
| Lymphocytes | −0.23* | 0.3 |
| CRP | 0.16* | 0.47 |
*Pearson correlation. , Mean±standard deviation. COVID-19=coronavirus disease 2019; HCV=hepatitis C virus; Hb=hemoglobin; CRP, C-reactive protein
| Lab Tests | Control group (n=35) | HD without COVID (n=156) | HD with COVID (n=23) | P | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| D--d Dimer | 542.76 | 107.06 | 1171.52 | 267.6 | <0.001 | ||
| TAT | 2.38 | 1.05 | 7.53 | 1.64 | 11.47 | 1.51 | <0.001 |
| P value (between groups) | <0.001a | ||||||
| <0.001b | |||||||
| <0.001c | |||||||
a; Control group versus ESKD without COVID group. b; Control group versus ESKD with COVID-19 group. c; COVID-19-positive versus COVID-19-negative group. TAT=thrombin–antithrombin complex; COVID-19=coronavirus disease 2019; HD=hemodialysis; SD=standard deviation
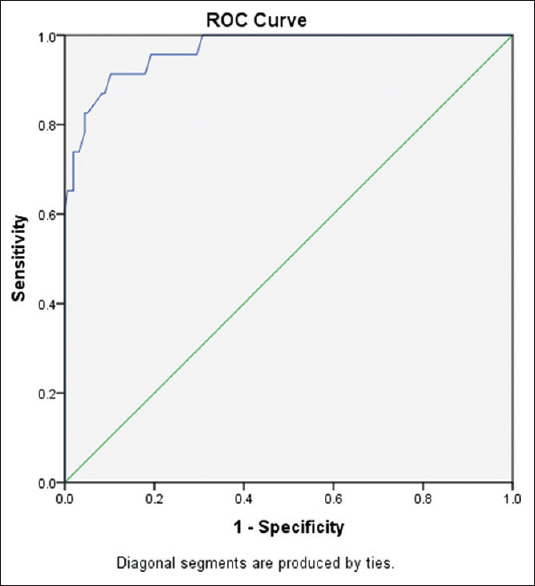
- The receiver operating characteristics (ROC) curve for thrombin–antithrombin complex levels in diagnosis of hypercoagulability state in COVID-positive end-stage renal disease patients
Discussion
Infection with COVID-19 has specific problems for HD patients, especially those receiving care in HD centers. A high percentage of HD patients were asymptomatic. It carries the risk of delayed proper management and the risk of spreading the infection as these asymptomatic patients are not isolated and there are almost about 6 days of the incubation period to develop the symptoms. The frequency of asymptomatic SARS-CoV-2 infection is higher than expected from PCR studies, and it only becomes apparent by additionally assessing the development of an immune response against the virus.[1] Asymptomatic HD patients may be more common and frequent than we predic.[10] It is difficult to estimate the actual prevalence of the asymptomatic, infected HD population without massive screening. Some studies showed that asymptomatic COVID-19 HD populations were estimated to be between 18% and 30%.[11,12] The number of people with COVID-19 decreased by more than 90% in 10 days when both symptomatic and asymptomatic patients were isolated.[10] So the importance of detecting asymptomatic patients lies in the ability of these patients to infect others.[8] One study showed that 15/90 (17%) HD patients with positive PCR were asymptomatic, representing 40% of those infected.[13] In a recent interesting study, 24 of 25 asymptomatic HD patients had abnormal lung computed tomography findings.[14] Another large-scale study in China suggested that 1.2% of patients had asymptomatic infection.[15] Whereas in another study conducted recently, 18.4% of HD patients had an asymptomatic COVID-19 infection.[3] COVID-19 infection is associated with increased thrombotic risks. It may even result in disseminated intravascular coagulation.[16] The problem lies in that not only COVID-19 but also its treatment alters the coagulation system. It is reported that extracorporeal membrane oxygenation (ECMO) can be an option for treatment in severe cases to support the cardiopulmonary function for some time till it regains its function.[17] Elevated levels of TAT and D-dimer along with comorbidities such as hypertension, diabetes, and cardiovascular disease carry a poor prognosis for COVID-19 patients.[18] Jin et al.[19] found that D-dimer values greater than 1.03 mg/L is a red flag that necessitates anticoagulant therapy. The difficulties of adopting epidemiological interventions such as isolation and social distancing in HD centers greatly increase the risk of the spread of infection. There is an apparent growth in COVID-19 infection in patients undergoing HD. In our study, we found that out of 179 HD patients, 23 (12.9%) of them tested positive for COVID-19 and 156 (87.1%) were negative by PCR. We found that D-dimer level was significantly higher in the COVID-19-positive group compared with the negative group. In a study conducted by Fisher et al.,[20] the authors reported a median (interquartile range [IQR]) D-dimer level of 0.3 (1.9–4.6) mg/mL. Ng et al.[21] in their study reported a median (IQR) D-dimer level of 583 (392–1090) ng/mL among positive COVID-19 patients. Moreover, we found that TAT level was also significantly higher in the COVID-19-positive group compared with other patients (11.47 ± 1.51 vs. 7.53 ± 1.64 mcq/L, respectively). This was much higher than that reported by Milburn et al.,[22] who reported TAT levels of 4.39 ± 1.07 and 2.83 ± 0.62 mcq/L in HD and non-HD groups, respectively. This is explained by the coincidence of COVID-19 infection and ESRD in our patients that greatly affects the TAT serum levels as TAT level is proved to be higher in ESRD patients undergoing HD. Our findings also showed values higher than that reported by Erdem et al.,[23] who reported TAT to be 4.5 ± 0.6 μg/dL in HD patients. However, this value greatly increased to be 11.9 ± 1.4 μg/dL when samples were collected from the peripheral blood. In our study, we found significantly higher levels of CRP among the positive group patients (52.5 ± 12.49 mg/dL), and this was higher than the level reported by Fisher et al.[20] and Ng et al.,[21] who reported 10.33 ± 4.37 mg/dL and 11.27 ± 4.13 mg/dL, respectively. The mean platelet count in the COVID-19-positive patients was 107.26 ± 29.54 × 109/L, which was significantly lower than the platelet count in the other group, and this was in contrast with Lano et al.,[24] who reported a platelet count of 170.5 ± 29.45 × 109/L in ESRD patients infected with COVID-19. Interestingly, a meta-analysis done by Lippi et al.[25] reported that the platelet count was inversely proportional to the severity of COVID-19 infection, and the patients in our study were asymptomatic and about 92% of patients in Lano et al. were symptomatic.[24] In our study, we found that there was a positive correlation between the D-dimer and CRP levels in the COVID-19-positive group. On the other hand, the platelet count shows a negative correlation with both D-dimer and TAT levels. We did not find a significant correlation between both D-dimer and TAT levels and the other variables such as age, duration of dialysis, hemoglobin, and lymphocytes. This may be due to the small number of cases in the positive group. To the best of our knowledge, no studies assessed the hypercoagulability state in asymptomatic COVID-19-positive ESRD patients undergoing HD.
The limitations of our study were the inability to follow up the patients. So further research work is recommended to conduct a study on larger number of patients and with long periods of follow-up to know the long-term sequelae of asymptomatic COVID-19 infection in the immunocompromised patients.
In conclusion, we found that HD patients are at risk for asymptomatic COVID-19 infection. Both D-dimer and TAT levels were higher in COVID-19-positive patients on regular HD. A study on large follow-up periods is required to examine the outcomes in the long term.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Clinical features of asymptomatic SARS-CoV-2 infection in hemodialysis patients. Kidney Blood Press Res. 2021;46:126-34.
- [Google Scholar]
- Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831-43.
- [Google Scholar]
- Clinical features of patients undergoing hemodialysis with COVID-19. Semin Dial. 2021;34:57-65.
- [Google Scholar]
- 2019 novel coronavirus disease in hemodialysis (HD) patients:Report from one HD center in Wuhan. China. 2020 https://doi.org/10.1101/20200.02.24.20027201.https://www.medrx?iv.org/conte?nt/early/?2020/02/27/2020.02.240.20027201
- [Google Scholar]
- Situación de la infección por SARS-CoV-2 enpacientes en tratamiento renal sustitutivo Informe del Registro COVID-19 de la Sociedad Española de Nefrología (S. E. N.) Nefrología. 2020;40:272-8.
- [Google Scholar]
- Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92:441-7.
- [Google Scholar]
- Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- [Google Scholar]
- Chinese guidelines related to novel coronavirus pneumonia. J Mark Access Heal Policy. 2020;8:1818446.
- [Google Scholar]
- Covid-19:Four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;369:m1375.
- [Google Scholar]
- Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan. Euro Surveill. 2020;25:2000180.
- [Google Scholar]
- Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154-5.
- [Google Scholar]
- Alta prevalencia de covid19 asintomático en hemodiálisis. Aprendiendo dia a dia el primer mes de pandemia de covid19. Nefrología. 2020;40:279-86.
- [Google Scholar]
- Asymptomatic patients with Novel Coronavirus Disease (COVID-19) Balkan Med J. 2020;37:229-30.
- [Google Scholar]
- The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chinese J Epidemiol. 2020;41:145-51.
- [Google Scholar]
- Using the big data of internet to understand the characteristics of coronavirus disease 2019:A big data study. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;55:569-75.
- [Google Scholar]
- Impact of AMICAR on hemorrhagic complications of ECMO:A ten-year review. J Pediatr Surg. 2003;38:1212-6.
- [Google Scholar]
- Prognostic genetic markers for thrombosis in COVID-19 patients:A focused analysis on D-Dimer, homocysteine and thromboembolism. Front Pharmacol. 2020;11:587451.
- [Google Scholar]
- The values of coagulation function in COVID-19 patients. PLoS One. 2020;15:e0241329.
- [Google Scholar]
- Chronic hemodialysis patients hospitalized with COVID-19:Short-term outcomes in the Bronx, New York. Kidney360. 2020;1:755-62.
- [Google Scholar]
- Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530-9.
- [Google Scholar]
- Thrombin-anti-thrombin levels and patency of arterio-venous fistula in patients undergoing haemodialysis compared to healthy volunteers:A prospective analysis. PLoS One. 2013;8:e67799.
- [Google Scholar]
- Coagulation, fibrinolysis and fibrinolysis inhibitors in haemodialysis patients:Contribution of arteriovenous fistula. Nephrol Dial Transplant. 1996;11:1299-305.
- [Google Scholar]
- Risk factors for severity of COVID-19 in chronic dialysis patients from a multicentre French cohort. Clin Kidney J. 2020;13:878-88.
- [Google Scholar]
- Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections:A meta-analysis. Clin Chim Acta. 2020;506:145-8.
- [Google Scholar]







